A new strategy for the greener use of calcium carbide
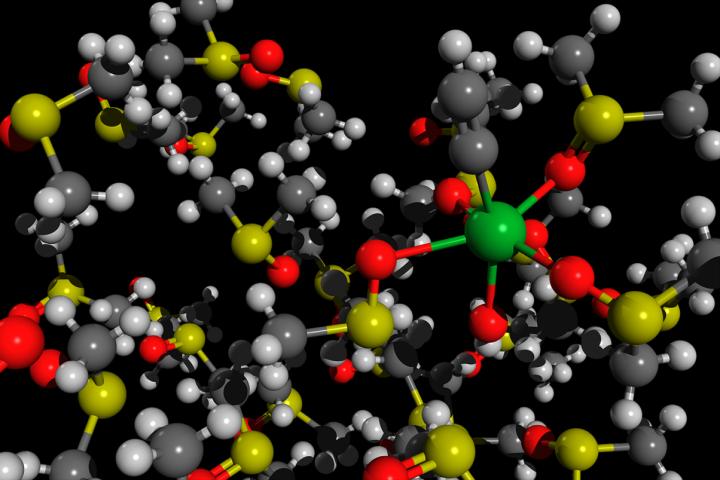
Born-Oppenheimer molecular dynamics of an acetylide molecule HO-Ca-CCH solvated in DMSO
Credit: Mikhail Polynski, assistant teaching fellow at the Institute of Chemistry of St Petersburg University
The researchers proposed a new approach by analysing the interaction of calcium acetylide with water and dimethyl sulfoxide on the atomic scale.
Calcium acetylide was discovered more than 150 years ago. It is a yellowish-white, beige, or grey solid, a compound of calcium and carbon. Calcium acetylide is currently used to produce gaseous acetylene. In industry, it is widely used in the production of acetic acid and ethyl alcohol, as well as it can be used in the production of plastics, rubber, and rocket engines.
The carbon needed for the synthesis of calcium acetylide is mined in an unsustainable way. As a result, the fossil resource is depleted (the unsustainable approach) and, furthermore, the amount of carbon increases above the earth’s surface. ‘We are working on a strategy for the carbon-neutral cycle of production. In particular, to obtain calcium acetylide, you can use carbon that is obtained via thermal decomposition (pyrolysis) of waste, and the resulting substance can be used in industry to create new compounds,’ said Konstantin Rodygin, a research associate at the Laboratory of Cluster Catalysis, St Petersburg University.
‘Nowadays, a key challenge for humanity is to create a new generation of industrial processes that make it possible to obtain the most important organic compounds and materials within the carbon-neutral approach. Of utmost importance is the replacement of fossil resources with renewable ones, thus solving environmental problems. As shown in our works, organic synthesis based on calcium acetylide opens up fresh opportunities for implementing carbon-neutral technologies. Moreover, understanding the chemical processes of the transformation of carbide species in chemical processes in solution is of key importance,’ said Valentin Ananikov, a member of the Russian Academy of Sciences and the Head of the Laboratories of Cluster Catalysis and Metal-Complex and Nanoscale Catalysts at the Zelinsky Institute.
The chemists were able to propose a new strategy for using the substance by simulating the processes that occur at the level of atoms and molecules during the interaction of calcium acetylide, water, and a dimethyl sulfoxide solvent. Calcium acetylide is a salt containing negatively charged acidic residues of acetylene (the so-called acetylide anions with a charge of ‘?2’) and positively charged calcium ions. The researchers investigated the acidity of acetylide anions, water, and some other substances in a dimethyl sulfoxide solvent. In that solvent, an unusual phenomenon can be observed; the interaction of acetylide anions with water, hydrolysis, proceeds incompletely. The so-formed anions, having a charge of ‘-1’, can undergo a wide range of key organic chemical reactions.
‘After performing the analysis, we realized that instead of water, one can use other protonating substances to transfer acetylide into solution. Also, one can potentially use a less toxic and more ‘green’ solvent instead of dimethyl sulfoxide to perform reactions involving calcium acetylide, if the solvent meets some newly-discovered criteria. Thus, chemical synthesis of new substances with calcium acetylide can be ‘greener’ due to the potential of calcium acetylide to react in less toxic solvents, as well as due to more sustainable approaches to the synthesis of the carbide itself’ said Mikhail Polynski, a co-author of the article, assistant teaching fellow at the Institute of Chemistry of St Petersburg University.
Noteworthy, one of the co-authors of the new article is Mariia Sapova, a graduate of St Petersburg University, who started work on the project during her master’s studies. ‘The challenging project attracted me from the start, as the idea of combining various computational methods opens up ample opportunities for the simulation of complex processes, such as, in our case, the dissolution process. This work gave me a broadened understanding of science in general and, moreover, encouraged me to go out of my comfort zone, modelling crystals, and to challenge the limits of applicability of various methods in computational chemistry. I think that methodologically complex modeling approaches like the proposed in the article should be developed further, so we can perform truly realistic modelling of chemical processes,’ noted Mariia Sapova.
As Mikhail Polynski specified, the newly presented research is purely theoretical; it is a computer simulation of the process of obtaining acetylides from calcium acetylide. ‘We used the so-called quantum chemical methods, Born-Oppenheimer molecular dynamics. As a result of such a simulation, it is possible to make a short molecular movie showing how the motion of atoms and molecules looks like at very short, picosecond, time scales,’ concluded Mikhail Polynski.
###
The research is a part of a project on the relevant chemistry of calcium acetylide funded by St Petersburg University. It is being carried out by the University’s Laboratory of Cluster Catalysis with the participation of researchers from the Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences. A significant part of the simulation was carried out using the facilities of the Computing Centre of the St Petersburg University Research Park.
Video from Ananikov Lab
Description: Born-Oppenheimer molecular dynamics of an acetylide molecule HO-Ca-CCH solvated in DMSO.
Author: Mikhail Polynski, assistant teaching fellow at the Institute of Chemistry of St Petersburg University
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Largest magnetic anisotropy of a molecule measured at BESSY II
At the Berlin synchrotron radiation source BESSY II, the largest magnetic anisotropy of a single molecule ever measured experimentally has been determined. The larger this anisotropy is, the better a…

Breaking boundaries: Researchers isolate quantum coherence in classical light systems
LSU quantum researchers uncover hidden quantum behaviors within classical light, which could make quantum technologies robust. Understanding the boundary between classical and quantum physics has long been a central question…

MRI-first strategy for prostate cancer detection proves to be safe
Active monitoring is a sufficiently safe option when prostate MRI findings are negative. There are several strategies for the early detection of prostate cancer. The first step is often a…



