A newly discovered enzyme makes ethylene and methane
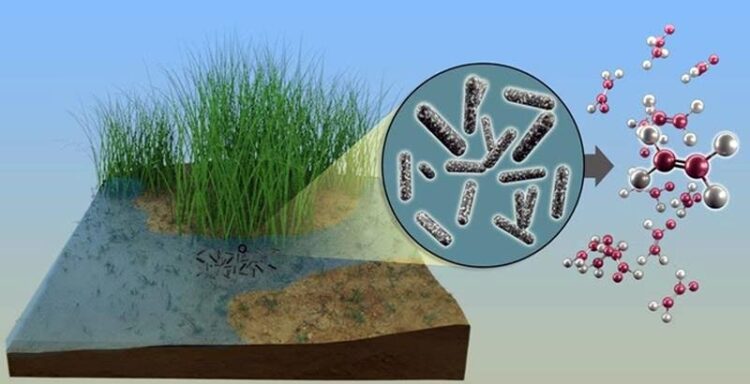
Oxygen-deprived bacteria in soil and freshwater environments make ethylene and methane as a byproduct of salvaging sulfur from small organic compounds.
Image courtesy of Andy Sproles, Oak Ridge National Laboratory
An enzyme system frees sulfur from small organic compounds to make a surprising gaseous side product.
The Science
Researchers often observe ethylene gas in oxygen-poor environments such as certain types of soil. For decades, scientists have known that ethylene comes from microbes. But the only known natural microbial processes that produce ethylene require oxygen. So how do oxygen-poor environments contain ethylene? A team of scientists have discovered an enzyme system from bacteria that produces ethylene without oxygen, shedding light on this paradox. These enzymes are called methylthio-alkane reductases. They function to scavenge sulfur from small volatile organic sulfur compounds. Depending on the compound used, these enzyme reactions produce ethylene, ethane, or methane as a byproduct.
The Impact
The newly discovered enzymes are a type of nitrogenases. Nitrogenases and their relatives are enzymes that perform chemical reactions requiring electrons. Scientists understand the role of nitrogenases and related substances in photosynthesis and methane formation. However, the function of many nitrogenase-like genes and related proteins in bacteria was a mystery. This study reveals that some of these relatives of nitrogenase have adapted specifically to break down sulfur. In the process, they release ethylene and methane. This highlights the unexplored functional diversity of nitrogenases. It also demonstrates their potential use in making biofuels and other biomaterials.
Summary
Ethylene is both a potent plant growth hormone as well as the key chemical precursor to most plastics, making it the most produced and used industrial chemical on the planet. Plants naturally produce ethylene at the proper time and amounts for root elongation, shoot extension, fruit ripening, and programmed leaf death. However, upon waterlogging and flooding, soils accumulate ethylene produced by microbes. This ethylene can accumulate to levels harmful to plants, leading to crop damage. Researchers previously discovered that some soil and freshwater bacteria can produce ethylene in the absence of oxygen when they are starved for sulfur. The researchers tracked down the pathway in bacteria that produces the immediate ethylene precursor, (2-methylthio)ethanol. But the final enzyme step that made ethylene remained unknown. Recently, a team of scientists uncovered this remaining step. Through a concerted series of genetic manipulations, proteomics, transcriptomics, and thermodynamic modeling, the team identified the methylthio-alkane reductases as the missing link. Subsequent metabolite labeling experiments and targeted metabolomics identified the reaction catalyzed. These reductases perform carbon-sulfur bond cleavage of (2-methylthio)ethanol to liberate the sulfur, releasing ethylene as a byproduct. Similarly, ethylmethyl sulfide and dimethyl sulfide can be used, releasing ethane and methane, respectively. The discovery of methylthio-alkane reductases provides an explanation for ethylene production in oxygen-deprived flooded soils and may provide insight into how to prevent detrimental ethylene accumulation. Additionally, biological ethylene formation in the absence of oxygen has potential advantages for industrial bio-ethylene production. Discovery of the methylthio-alkane reductases completes the oxygen-independent ethylene producing pathway, paving the way for engineering bacteria strains that produce high levels of ethylene.
Funding
This material is based on work supported by the Department of Energy (DOE) Office of Science, Office of Biological & Environmental Research, an Ohio State University Center for Applied Plant Sciences Grant, and the National Science Foundation. Electronic structure calculations were performed by the Environmental Molecular Sciences Laboratory, a user facility sponsored by DOE at Pacific Northwest National Laboratory. Transcriptomics work was supported in part by the University of Colorado Cancer Center’s Genomics and Microarray Shared Resource, which is supported by the National Cancer Institute. Proteomics work at Oak Ridge National Laboratory was supported by the DOE Office of Science Genomic Science Program as part of the Plant Microbe Interfaces Scientific Focus Area.
Journal: Science
DOI: 10.1126/science.abb6310
Method of Research: Experimental study
Subject of Research: Not applicable
Article Title: A nitrogenase-like enzyme system catalyzes methionine, ethylene, and methane biogenesis
Article Publication Date: 28-Aug-2020
Media Contact
Michael Church
DOE/US Department of Energy
michael.church@science.doe.gov
Office: 2028416299
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



