A quantum spin on molecular computers
One day powerful quantum computers could be made from molecules like these -- vanadium complexes. Credit: American Chemical Society
Traditional computers rely on transistors that occupy one of two states — that's what those archetypal zeroes and ones refer to, and each digit is a “bit.” Quantum computing would use three states, improving its information storage capacity exponentially.
Whereas a small app like “Angry Birds” takes up about 40,000 standard bits, a computer made with just 1,000 quantum bits, or “qubits,” could easily and quickly break modern encryption schemes or more precisely model how a pharmaceutical drug candidate would perform in a person. The biggest challenge of quantum computing, however, is making the qubit.
Some of the most promising qubits today use electrons, specifically their “spin” state. Spin can have two states, just like a bit, but also a combination of both to form a third state, called “superposition.” But very few molecules stay in the superposition state long enough to measure, which makes them difficult to use in computing.
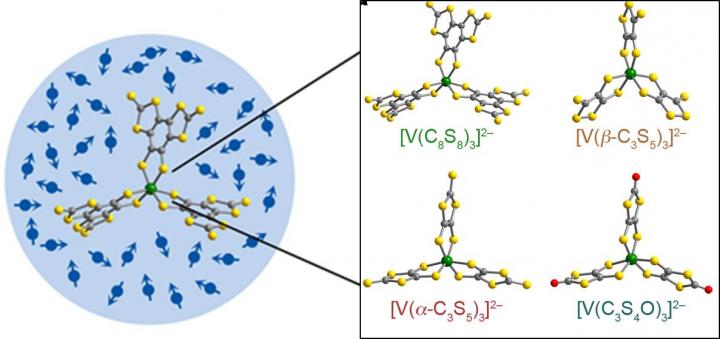
One reason is that the interaction of spins on most nuclei can interfere with the electronic ones. To get closer to a real, functional qubit, Danna Freedman and colleagues turned to metal complexes, where most of those problematic nuclear spins were eliminated.
Freedman and colleagues synthesized vanadium complexes with arms made of carbon and sulfur. As long as the system was kept cold, these molecules kept superposition longer than any metal complexes previously reported. They also kept that state for just as long as other bulk materials currently under consideration.
These new molecules show that under the right conditions, inorganic complexes can function as viable qubits. In addition, the complexes may prove to be superior to other potential materials because their defined chemical structure could more easily allow the organized design of functional devices. To get a little meta: it's possible that one day computers made of just a handful of small molecules will be used to make predictions about other molecules.
The authors acknowledge funding from Northwestern University, the State of Illinois, the National Science Foundation and the Department of Energy.
The paper will be freely available on Dec. 2 at this link: http://pubs.
The American Chemical Society is a nonprofit organization chartered by the U.S. Congress. With more than 158,000 members, ACS is the world's largest scientific society and a global leader in providing access to chemistry-related research through its multiple databases, peer-reviewed journals and scientific conferences. Its main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive news releases from the American Chemical Society, contact newsroom@acs.org.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative vortex beam technology
…unleashes ultra-secure, high-capacity data transmission. Scientists have developed a breakthrough optical technology that could dramatically enhance the capacity and security of data transmission (Fig. 1). By utilizing a new type…

Tiny dancers: Scientists synchronise bacterial motion
Researchers at TU Delft have discovered that E. coli bacteria can synchronise their movements, creating order in seemingly random biological systems. By trapping individual bacteria in micro-engineered circular cavities and…

Primary investigation on ram-rotor detonation engine
Detonation is a supersonic combustion wave, characterized by a shock wave driven by the energy release from closely coupled chemical reactions. It is a typical form of pressure gain combustion,…



