A Rapid Extension of Nanographene Sheets from Readily Available Hydrocarbons
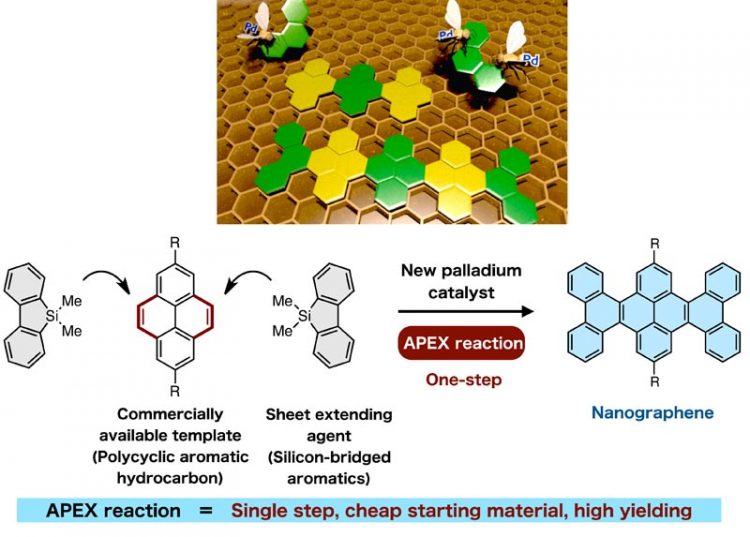
Selective annulation at the ‘K-region’ leads to direction-controlled synthesis of nanographenes in high yield. Copyright : WPI-ITbM, Nagoya University
The rapid and uniform construction of nanographene sheets has now become possible in a precisely controlled manner from a new catalytic system developed by a team of chemists at the Institute of Transformative Bio-Molecules (ITbM), Nagoya University and the JST-ERATO Project led by Professor Kenichiro Itami.
Commercially available hydrocarbons were used as templates to synthesize uniform nanographene sheets using a series of sheet extending agents and a new transition metal catalyst, which were developed by Kyohei Ozaki, Katsuaki Kawasumi, Mari Shibata, Hideto Ito, and Professor Kenichiro Itami at Nagoya University’s Institute of Transformative Bio-Molecules (ITbM) and of the JST-ERATO Itami Molecular Nanocarbon Project. Nanographenes, which are nanometer-sized graphenes, possess good semiconducting properties making them highly promising materials for the next generation of organic electronic devices.
Itami and his team have developed a new catalytic one-shot annulative π (pi)-extension (APEX) reaction, which enables the rapid construction of nanographene in a single-step. The study, published online on February 16, 2015 in Nature Communications, illustrates the discovery of efficient sheet extending agents and a highly reactive palladium catalyst, making the APEX reaction highly applicable towards the ‘growth from template’ construction of a variety of nanographene structures as well as fine-tuning of their properties.
The physical properties of nanographenes are mainly determined by their width, length and edge structures. Therefore, structural control on a nanometer-scale is highly desirable to access useful nanographenes. Many nanographene syntheses reported up to now involve a two-step sequence of (i) assembly of small aromatic components, followed by (ii) stitching (graphenization) of polycyclic precursors to make them into sheets. Although these methodologies have been useful, multiple steps are required for the assembly of molecules.
In addition, incomplete stitching, side reactions and low yields have caused difficulty in the precise control for the synthesis of nanographenes. To overcome this, Itami and his co-workers have proposed a single-step APEX reaction, which involves selective attachment and annulation of an aromatic π-extending agent on a specific region of readily available polycyclic aromatic hydrocarbons (PAHs).
Up until now, there have been no reported examples of APEX reactions conducted at the convex armchair edge of PAHs, otherwise known as the K region. Reactions at the K-region are considered to be difficult as other C-H positions are usually more prone to aromatic substitution reactions.
“We started to work on developing APEX reactions when we found a palladium catalyst that led to selective arylation (attachment of an aromatic group) at the K-region,” says Itami, the Director of ITbM and the JST-ERATO project. “After stitching, we were able to synthesize warped nanographenes in two-steps. Based on this insight, we envisaged that we could selectively activate the K-region and induce cyclization in one-step by careful choice of catalysts and reactants.”
“As far as we know, there have been no examples on the use of PAHs as starting materials for the synthesis of nanographenes,” says Ito, a co-author of this study. “Although PAHs are relatively cheap and readily available, their relatively low reactivity and difficulty in selectively installing functionalities have limited their use as substrates,” he adds. “Therefore, we carried out extensive screening of palladium salts, additives, and sheet (π)-extending agents to find the right combination of reagents that induced the APEX reaction on PAHs to generate uniform sheets of nanographene.”
“The most surprising part of this research was that a silicon-bridged aromatic compound could be used as a sheet extending agent for the APEX reaction,” says Ozaki and Kawasumi, graduate students of Nagoya University who conducted the experiments. “The silicon-bridged molecule was a product of a reaction, in which we were actually expecting a different product. Because we had the molecule in hand, we decided to use it in the catalytic system and coincidently found that selective annulation at the K-region had occurred.”
In order to investigate the nature of this APEX reaction, computational studies using density functional theory calculations were conducted on a model reaction. “We predict that the selectivity of the APEX reaction arises from the K-region π-bond having the least aromatic character compared to other sites,” says Shibata, who performed the theoretical calculations. “Further computational studies will be carried out to find more details on what is actually happening in the APEX reaction.”
Functional handles, such as chloride and boryl groups on the substrates were compatible under the APEX reaction conditions, which makes the products ready for further functionalization. The synthetic utility of the APEX reaction was also demonstrated by examining multiple and sequential APEX reactions to successfully access larger nanographene frameworks on a multi-gram scale. “The most difficult part of this research was by far the solubility issue that we had with handling the nanographene products,” says Ozaki and Ito. “Most of them were insoluble in the solvents that we tried. However, I was sure that I could make this reaction better and I believe that it was my determination to accomplish the precisely controlled synthesis of nanographenes that finally led to this outcome,” continues Ozaki.
“Through the development of the APEX reaction, we have succeeded in the direction-controlled growth of nanographene sheets from PAHs,” says Itami. “We believe that this methodology will lead to the synthesis of uniform nanographenes that are essential for future nanomaterial devices, and will also be applicable for constructing other exciting conjugated structures in a rapid and programmable manner.”
This article “One-shot K-region-selective annulative π-extension for nanographene synthesis and functionalization” by Kyohei Ozaki, Katsuaki Kawasumi, Mari Shibata, Hideto Ito and Kenichiro Itami is published online on February 16, 2015 in Nature Communications.
DOI: 10.1038/ncomms7251 (http://www.dx.doi.org/10.1038/ncomms7251)
About WPI-ITbM (http://www.itbm.nagoya-u.ac.jp/)
The World Premier International Research Center Initiative (WPI) for the Institute of Transformative Bio-Molecules (ITbM) at Nagoya University in Japan is committed to advance the integration of synthetic chemistry, plant/animal biology and theoretical science, all of which are traditionally strong fields in the university. As part of the Japanese science ministry’s MEXT program, ITbM aims to develop transformative bio-molecules, innovative functional molecules capable of bringing about fundamental change to biological science and technology. Research at ITbM is carried out in a “Mix-Lab” style, where international young researchers from multidisciplinary fields work together side-by-side in the same lab. Through these endeavors, ITbM will create “transformative bio-molecules” that will dramatically change the way of research in chemistry, biology and other related fields to solve urgent problems, such as environmental issues, food production and medical technology that have a significant impact on the society.
About JST-ERATO Itami Molecular Nanocarbon Project (http://www.jst.go.jp/erato/itami/index.html)
This project entails the design and synthesis of as-yet largely unexplored nanocarbons as structurally well-defined molecules, and the development of novel, highly functional materials based on these nanocarbons. Through the combination of chemical and physical methods, the project aims to achieve the controlled synthesis of well-defined uniquely structured nanocarbon materials. Interdisciplinary research is conducted to encompass the control of molecular arrangement and orientation, structural and functional analysis, and applications in devices and biology.
About JST-ERATO (http://www.jst.go.jp/erato/en/about/index.html)
ERATO (The Exploratory Research for Advanced Technology), one of the Strategic Basic Research Programs, aims to form a headstream of science and technology, and ultimately contribute to science, technology, and innovation that will change society and the economy in the future. In ERATO, a Research Director, a principal investigator of ERATO research project, establishes a new research base in Japan and recruits young researchers to implement his or her challenging research project within a limited time frame.
Author Contact
Professor Kenichiro Itami
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL/FAX: +81-52-788-6098
E-mail: itami@chem.nagoya-u.ac.jp
About ERATO Program
Takeshi Ohyama
Department of Research Project, Japan Science and Technology Agency (JST)
K’s Goban-cho, 7 Goban-cho, Chiyoda-ku Tokyo 102-0076, Japan
TEL: +81-3-3512-3528 FAX: +81-3-3222-2068
E-mail: eratowww@jst.go.jp
Media Contact
Dr. Ayako Miyazaki
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL: +81-52-789-4999 FAX: +81-52-789-3240
E-mail: press@itbm.nagoya-u.ac.jp
Nagoya University Public Relations Office
TEL: +81-52-789-2016 FAX: +81-52-788-6272
E-mail: kouho@adm.nagoya-u.ac.jp
Associated links
http://www.dx.doi.org/10.1038/ncomms7251
Journal information
Nature Communications
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



