Cell receptors: of voids and void fillers
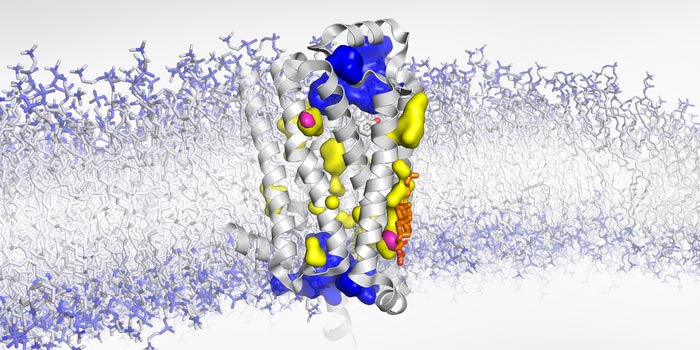
Structure of the membrane-bound β1-adrenergic receptor with water-exposed cavities (blue), not accessible to water (yellow), and dry voids (magenta).
Credit: Biozentrum, University of Basel
Nearly all vital functions in the human body are regulated by so-called G protein-coupled receptors on the cell surface. These receptors thus serve as attractive drug targets to treat various diseases. Researchers led by Prof. Stephan Grzesiek from the Biozentrum, University of Basel, have now discovered that empty spaces inside these receptors are important for their activation and thus for relaying messages to the inner cell. Their approach to locate these voids may help to direct the search for novel drugs.
The G protein-coupled receptors (GPCRs) enable us to see, taste food, feel cold or warm, or respond to stress, among other things. Located on the cell surface, GPCRs sense a large variety of signals such as nutrients, light, odors or hormones. By changing their conformation, they transmit this information from the outside to the inside of the cell. The accumulated knowledge about GPCRs has tremendously affected modern medicine: about one-third of all marketed drugs target GPCRs.
Empty spaces important for receptor activation
Using cutting-edge technology, the research team led by Prof. Stephan Grzesiek, together with collaborators at the Biozentrum of the University of Basel and the Paul Scherrer Institute, has now discovered that GPCRs contain completely empty cavities which are important for their activation. Their recent, experimental approach, published in Nature Chemistry, may direct and speed up the search for new and more specific drug candidates with fewer side effects.
Although the 826 GPCRs within the human body respond to many different stimuli, they all share a common architecture. “Our aim is to understand at the atomic level how GPCRs transmit signals,” says Dr. Layara Abiko, who co-directed the study. “For many years, we have therefore been studying the β1-adrenergic receptor, a GPCR that prepares the body for fight or flight.” The hormone adrenaline binds to and activates the receptor which triggers a stress response, for example, causing an increase in heart rate and blood pressure.” Beta-blockers inhibit this receptor and thus are effective drugs to treat hypertension or cardiovascular diseases.
Exact localization of dry voids
“Thanks to high-pressure NMR and our experimental approach using X-ray scattering on receptor crystals that incorporated the noble gas xenon, we could further complete the picture of this highly dynamic receptor,” says Abiko. ”Previously, it was assumed that the cavities inside the receptor are filled with water. We have now revealed that some of them are empty.” During activation, the conformation of the receptor changes in such a way that these dry voids get compressed and disappear. Consequently, the receptor shrinks just like when you squeeze a sponge. In case of the β1-adrenergic receptor, this conformational change is key for initiating the body’s fight-or-flight response.
The researchers have now been able to exactly localize two of such empty cavities and revealed that cholesterol – an important cell membrane component – can fill one of these. Like a wedge, cholesterol impedes the receptor from squeezing and changing to its fully active state. “Blocking this void obstructs the subtle, but essential movements required to activate the GPCR,” explains Abiko. “We think, this wedge effect could be another layer of receptor regulation.”
New routes for drug development
But why can scouting for dry voids be important? Classical drug binding sites are often similar among GPCR subclasses. A drug directed to such a site may bind to more than one receptor and therefore cause unwanted side effects. In contrast, the dry cavities differ considerably between GPCRs, even when they are from the same subclass. This makes them highly selective drug targets.
“In this way, you may design drugs that are highly specific for one receptor”, explains Abiko. The developed new approach may locate such unconventional drug binding sites which differ strongly between the receptors. This can help the screening process for new therapeutics, save time and reduce costs.
Journal: Nature Chemistry
DOI: 10.1038/s41557-022-01009-9
Article Title: Filling of a water-free void explains the allosteric regulation of the β1-adrenergic receptor by cholesterol.
Article Publication Date: 11-Aug-2022
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



