Chemical synthesis of nanotubes
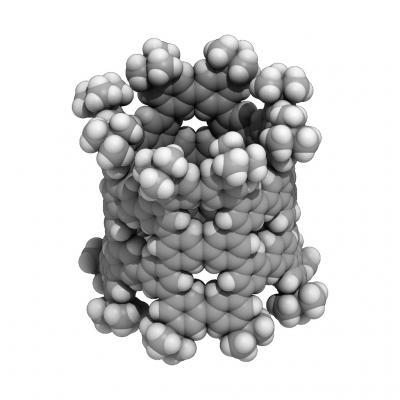
A nanometer-sized pNT cylinder made of 40 benzenes. The cylinder is tens of thousands of times thinner than a human hair. Credit: (c)2018 Hiroyuki Isobe
For the first time, researchers used benzene – a common hydrocarbon – to create a novel kind of molecular nanotube, which could lead to new nanocarbon-based semiconductor applications.
Researchers from the Department of Chemistry have been hard at work in their recently renovated lab in the University of Tokyo's Graduate School of Science.
The pristine environment and smart layout affords them ample opportunities for exciting experiments. Professor Hiroyuki Isobe and colleagues share an appreciation for “beautiful” molecular structures and created something that is not only beautiful but is also a first for chemistry.
Their phenine nanotube (pNT) is beautiful to see for its pleasing symmetry and simplicity, which is a stark contrast to its complex means of coming into being. Chemical synthesis of nanotubes is notoriously difficult and challenging, even more so if you wish to delicately control the structures in question to provide unique properties and functions.
Typical carbon nanotubes are famous for their perfect graphite structures without defects, but they vary widely in length and diameter. Isobe and his team wanted a single type of nanotube, a novel form with controlled defects within its nanometer-sized cylindrical structure allowing for additional molecules to add properties and functions.
The researchers' novel process of synthesis starts with benzene, a hexagonal ring of six carbon atoms. They use reactions to combine six of these benzenes to make a larger hexagonal ring called a cyclo-meta-phenylene (CMP). Platinum atoms are then used which allow four CMPs to form an open-ended cube.
When the platinum is removed, the cube springs into a thick circle and this is furnished with bridging molecules on both ends enabling the tube shape.
It sounds complicated, but amazingly, this complex process successfully bonds the benzenes in the right way over 90 percent of the time. The key also lies in the symmetry of the molecule, which simplifies the process to assemble as many as 40 benzenes.
These benzenes, also called phenines, are used as panels to form the nanometer-sized cylinder. The result is a novel nanotube structure with intentional periodic defects. Theoretical investigations show these defects imbue the nanotube with semiconductor characters.
“A crystal of pNT is also interesting: The pNT molecules are aligned and packed in a lattice rich with pores and voids,” Isobe explains. “These nanopores can encapsulate various substances which imbue the pNT crystal with properties useful in electronic applications. One molecule we successfully embedded into pNT was a large carbon molecule called fullerene (C70).”
“A team lead by Kroto/Curl/Smalley discovered fullerenes in 1985. It is said that Sir Harold Kroto fell in love with the beautiful molecule,” continues Isobe. “We feel the same way about pNT. We were shocked to see the molecular structure from crystallographic analysis. A perfect cylindrical structure with fourfold symmetry emerges from our chemical synthesis.”
“After a few decades since the discovery, this beautiful molecule, fullerene, has found various utilities and applications,” adds Isobe. “We hope that the beauty of our molecule is also pointing to unique properties and useful functions waiting to be discovered.”
###
Journal article
Zhe Sun, Koki Ikemoto, Toshiya M. Fukunaga, Takashi Koretsune, Ryotaro Arita, Sota Sato and Hiroyuki Isobe. Finite phenine nanotubes with periodic vacancy defects. Science. DOI:10.1126/science.aau5441
Related links
Laboratory of Physical Organic Chemistry – http://www.
Department of Chemistry – http://www.
Graduate School of Science – https:/
ERATO Isobe Degenerate π-Integration Project – http://www.
Research Contact
Professor Hiroyuki Isobe
Department of Chemistry, Graduate School of Science, The University of Tokyo
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 JAPAN
Tel: +81-3-5841-4777
Email: isobe@chem.s.u-tokyo.ac.jp
Press Contacts
Ms. Kristina Awatsu
Office of Communication, Graduate School of Science, The University of Tokyo
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033 JAPAN
Tel: +81-3-5841-8737
E-mail: kouhou.s@gs.mail.u-tokyo.ac.jp
Mr. Rohan Mehra
Division for Strategic Public Relations, The University of Tokyo
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8654 JAPAN
Tel: +81-3-5841-0876
Email: press-releases.adm@gs.mail.u-tokyo.ac.jp
About the University of Tokyo
The University of Tokyo is Japan's leading university and one of the world's top research universities. The vast research output of some 6,000 researchers is published in the world's top journals across the arts and sciences. Our vibrant student body of around 15,000 undergraduate and 15,000 graduate students includes over 2,000 international students. Find out more at https:/
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



