Communication between cells plays a major role in deciding their fate
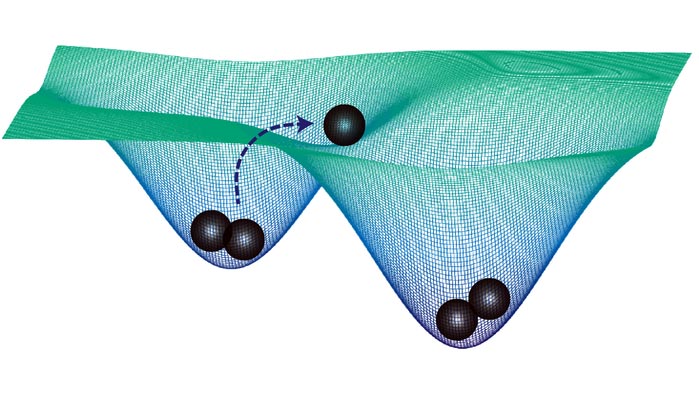
In this schematic, cells (black spheres) within each well are committed to a specific fate, but external stimuli, such as cell-to-cell communication, can force cells out of one state and into another.
Credit: Adam MacLean
Findings from a new study could point the way to new treatments for blood diseases including cancers such as leukemia and lymphoma.
Scientists have found a way to prove that biochemical signals sent from cell to cell play an important role in determining how those cells develop.
The study from researchers at the USC Dornsife College of Letters, Arts and Sciences was published in the journal Development on Dec. 22.
A little background:
- All cells within the body begin as stem cells.
- In simplest terms, blood forms when stem cells in bone marrow develop along one of three paths to become either oxygen-carrying red cells, immune-system white cells, or platelets, which clot to stop bleeding.
- Scientists have long accepted that communication between cells can affect their fate, but they have largely found it too complex to study directly.
- Cells can communicate by sending growth factors, hormones or other molecules back and forth.
What’s new:
- USC Dornsife’s Adam MacLean, assistant professor of quantitative and computational biology, and doctoral candidate Megan Rommelfanger, found a way to better understand how cell-to-cell communication affects the way blood stem cells develop.
- The scientists discovered that the communication process can change the formation of blood cell types dramatically.
- They also found that distance between cells matters.
“We discovered that the communication process can change the formation of blood cell types dramatically, and that cells that are closer to one another have a greater influence on each other’s fate,” MacLean said.
A controversy resolved
Researchers trying to determine what early factors nudge a cell down one developmental path or another have wondered if random fluctuations within the cell are enough to decide which path is taken. Many models have suggested they were, but recent breakthrough studies showed that random fluctuations were not enough, that something else drives cells toward their fate.
The model MacLean and Rommelfanger have developed appears to put an end to the controversy altogether. They show that cell-to-cell communication can, in fact, be the deciding factor that sets cells along a certain path.
Why it matters:
- While focusing on understanding stem cells, the research can help scientists understand how cancer arises.
- Leukemias, for example, develop when certain white blood cells begin growing and accumulating out of control.
- Determining the factors that push cells down the path to cancer can open avenues for prevention and therapy.
“By understanding how blood cell fate decisions are made,” MacLean said, “we get closer to being able to identify leukemia cells of origin, and in theory we can design strategies to control or alter cell fate decision-making and stop the development of cancer.”
The research could help improve cancer therapies such as bone marrow transplant.
- Bone marrow transplant involves infusing marrow — and the stem cells within — from a healthy donor into leukemia and lymphoma patients whose cancer and marrow has been obliterated by chemotherapy and radiation.
- Despite its success, scientists and clinicians still aren’t fully sure how BMT works.
“Better understanding stem cell fate decisions, as our study provides, could provide new insight to improve clinical outcomes for these diseases,” MacLean said.
More than just blood
This new model has important implications beyond the blood system.
“Our model is broadly applicable, so researchers working on other cell types can apply it to find out for those other cells how important cell-to-cell communication may be,” said MacLean.
What’s next:
The role of cell-to-cell communication in determining cell fate is in its nascent stages, says MacLean, but further experiments — and future technologies to integrate these new types of data with sophisticated models — should help expand understanding.
In addition, the team is developing methods to study the regulation of key genes involved in cell fate decisions, which should further advance their overall theoretical model.
About the study
This work was supported by National Science Foundation grant DMS 2045327 and a USC Women in Science and Engineering Top-up Fellowship.
Journal: Development
DOI: 10.1242/dev.199779
Method of Research: Computational simulation/modeling
Subject of Research: Cells
Article Title: A single-cell resolved cell-cell communication model explains lineage commitment in hematopoiesis
Article Publication Date: 22-Dec-2021
COI Statement: The authors declare no competing or financial interests.
Media Contact
James Key
University of Southern California
jameskey@usc.edu
Cell: 619-253-1077
Original Source
https://dornsife.usc.edu/news/stories/3609/cell-fate-development-cell-to-cell-communication/
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Skull bone marrow expands throughout life
…and remains healthy during aging. Blood vessels and stromal cells in the bone marrow create an ideal environment for hematopoietic stem cells to continuously produce all blood cells. During aging,…

Future AR/VR controllers could be the palm of your hand
Carnegie Mellon University’s EgoTouch creates simple interfaces for virtual and augmented reality. The new generation of augmented and virtual reality controllers may not just fit in the palm of your…

‘Game changer’ in lithium extraction
Rice researchers develop novel electrochemical reactor. A team of Rice University researchers led by Lisa Biswal and Haotian Wang has developed an innovative electrochemical reactor to extract lithium from natural…



