Copper hydroxide nanoparticles provide protection against toxic oxygen radicals in cigarette smoke
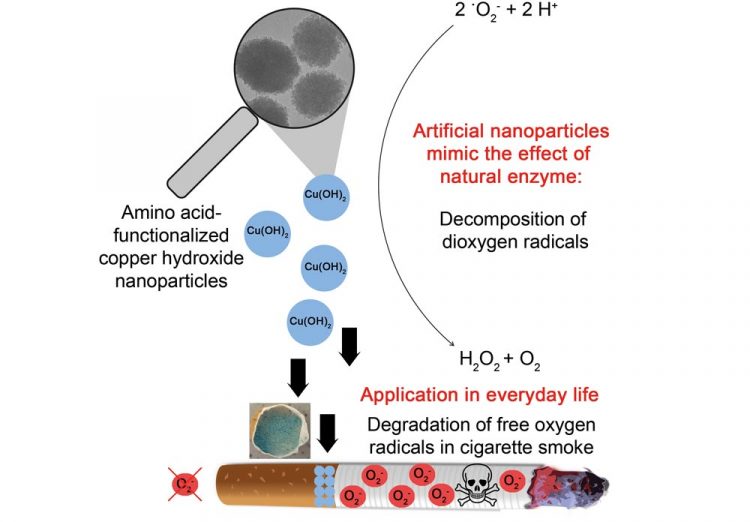
From laboratory to everyday life: Artificially produced copper hydroxide nanoparticles catalyze the decomposition of oxygen radicals by imitating a natural enzyme-induced catalytic defense mechanism. The integration of such nanoparticles in commercial cigarette filters can result in a reduction of the toxicity of cigarette smoke. Ill./©: Karsten Korschelt, AG Tremel, JGU
Chemists at Johannes Gutenberg University Mainz (JGU) have developed a technique that reduces the toxic effects of commercially available cigarettes. In spite of the fact that the World Health Organization (WHO) estimates that some 6 million people die every year as a consequence of tobacco consumption, the number of smokers around the world is on the rise.
The number of tobacco-related deaths is equivalent to the fatality rate that would occur if a passenger plane were to crash every hour. According to figures published by the German Federal Statistical Office, the tobacco industry generated a turnover of around EUR 20.5 billion in 2016 through the sale of cigarettes in Germany alone.
Tobacco smoke contains almost 12,000 different constituents. Among these are narcotoxic substances such as nicotine, blood toxins like cyanide and carbon monoxide, not to mention the various carcinogens. Among these are free oxygen radicals, also known as reactive oxygen species. More than 10 quadrillion (10^16) of these molecules are inhaled with every puff on a cigarette.
The Mainz-based team headed by Professor Wolfgang Tremel has discovered how to significantly lower the levels of these free oxygen radicals and thus markedly reduce the toxicity of cigarette smoke. This development could help not only to make the consumption of tobacco-based products somewhat less hazardous but it could also be extended to other areas in which reactive oxygen radicals are a problem.
Researchers took the underlying idea behind the concept from natural enzymes. In the presence of an enhanced concentration of reactive oxygen species as a result of, for instance, enzymatic dysfunction, UV radiation or the inhalation of tobacco smoke, uncontrolled cell division and oxidative cell damage can occur.
Nature regulates the concentration of radicals by means of antioxidant enzymes such as superoxide dismutase (SOD), which plays a central role in the prevention of pathological processes, including tumor and cancer growth, inflammatory diseases, and stroke. The naturally occurring enzyme utilizes metals such as copper-zinc, nickel, iron, and manganese as reactive centers that cause oxygen radicals to decompose so that the organism is protected from their aggressive reactive behavior.
Today it is possible to produce or isolate enzymes like SOD, but the process is accompanied by high costs. However, their poor stability on exposure to high temperatures and non-physiological pH values complicates matters. With natural enzymes in mind, researchers in the field of biomimetics are seeking ways to imitate natural biological reactions with the help of synthetic compounds.
Chemist Karsten Korschelt and food chemist Dr. Carmen Metzger investigated amino acid-functionalized copper hydroxide nanoparticles as potential synthetic analogues of cupriferous SOD. They found that the particles were associated with a higher rate of catalytic activity in terms of the decomposition of oxygen radicals than the enzyme itself. “This is in principle not such a surprise as all copper atoms on the particle surface can have a catalytic effect, yet the enzyme has only one active center,” said Professor Wolfgang Tremel. In contrast to natural enzymes, functionalized copper hydroxide nanoparticles are very stable and inexpensive to produce.
From laboratory to everyday life
Although natural enzymatic reactions can be mimicked with the help of nanoparticles, there are as yet only a few applications based on the principle. Nanoparticles themselves are used in cosmetics, for instance, and as nano sealants in paints and textiles. We are so extensively exposed to free radicals on a day-to-day basis that their presence is frequently ignored altogether.
But in exhaust fumes and cigarette smoke they represent a major threat to health. The team of chemists in Mainz is thus collaborating with the group headed by Professor Jürgen Brieger of the Mainz University Medical Center in order to determine whether it is possible to integrate functionalized copper hydroxide nanoparticles in cigarette filters and thus reduce levels of free radicals in smoke, hence providing smokers with greater protection against their toxic potential.
Cytotoxicity tests have shown that the cigarette smoke extracts in the examined concentrations no longer have a toxic effect on human cells after passing through cigarette filters containing nanoparticles, while there was increased toxicity in the case of controls in which untreated filters were employed.
“This demonstrates the positive effect of the particles when used in cigarette filters and their stability during the smoking process,” emphasized Karsten Korschelt. The researchers in Mainz have thus been able to demonstrate that imitating natural defense mechanisms with the help of nanoparticles is possible and that a reduction in the toxic effects of various types of smoke can be achieved.
The employed methods and analyses were developed and implemented in cooperation with the University Medical Center of Johannes Gutenberg University Mainz. The report on their work has been published in the scientific journal Nanoscale.
Original publication:
Karsten Korschelt et al.
Glycine-Functionalized Copper(II) Hydroxide Nanoparticles with High Intrinsic Superoxide Dismutase Activity
Nanoscale, 22 February 2017
DOI: 10.1039/C6NR09810J
Caption:
http://www.uni-mainz.de/bilder_presse/09_anorgchemie_artificial_copper_hydroxide…
From laboratory to everyday life: Artificially produced copper hydroxide nanoparticles catalyze the decomposition of oxygen radicals by imitating a natural enzyme-induced catalytic defense mechanism. The integration of such nanoparticles in commercial cigarette filters can result in a reduction of the toxicity of cigarette smoke.
Ill./©: Karsten Korschelt, AG Tremel, JGU
Contact and further information:
Professor Dr. Wolfgang Tremel
Institute of Inorganic Chemistry and Analytical Chemistry
Johannes Gutenberg University Mainz
55099 Mainz, GERMANY
phone +49 6131 39-25135
fax +49 6131 39-25605
e-mail: tremel@uni-mainz.de
http://www.ak-tremel.chemie.uni-mainz.de/index.php
http://pubs.rsc.org/en/content/articlelanding/2017/nr/c6nr09810j#!divAbstract – Article in Nanoscale ;
https://www.chemistryworld.com/news/nanoparticles-mimic-enzyme-to-reduce-toxins-… – Article in Chemistry World
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Breaking the Ice: Glacier Melting Alters Arctic Fjord Ecosystems
The regions of the Arctic are particularly vulnerable to climate change. However, there is a lack of comprehensive scientific information about the environmental changes there. Researchers from the Helmholtz Center…

Global Genetic Insights into Depression Across Ethnicities
New genetic risk factors for depression have been identified across all major global populations for the first time, allowing scientists to predict risk of depression regardless of ethnicity. The world’s…

Back to Basics: Healthy Lifestyle Reduces Chronic Back Pain
Low back pain is a leading cause of disability worldwide with many treatments, such as medication, often failing to provide lasting relief. Researchers from the University of Sydney’s Centre for Rural…



