Crystal clear images uncover secrets of hormone receptors
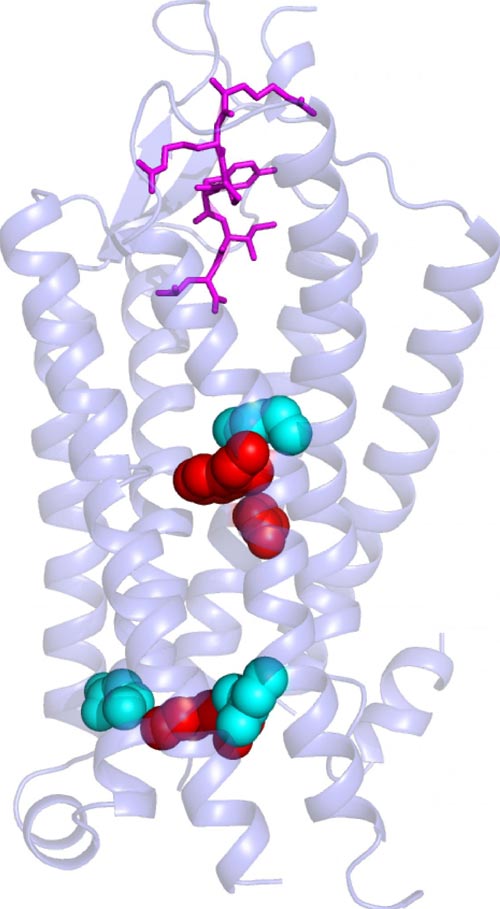
NIH scientists described for the first time how a neuropeptide hormone may activate a G protein-coupled receptor. Courtesy of Grisshammer lab, NINDS, Bethesda, Md.
Many hormones and neurotransmitters work by binding to receptors on a cell's exterior surface. This activates receptors causing them to twist, turn and spark chemical reactions inside cells. NIH scientists used atomic level images to show how the neuropeptide hormone neurotensin might activate its receptors. Their description is the first of its kind for a neuropeptide-binding G protein-coupled receptor (GPCR), a class of receptors involved in a wide range of disorders and the target of many drugs.
“G protein-coupled receptors are found throughout the body. Knowing how they work should help scientists devise better treatments,” said Reinhard Grisshammer, Ph.D., an investigator at the NIH's National Institute of Neurological Disorders and Stroke (NINDS) and the senior author of the study published in Nature Communications.
Neurotensin is thought to be involved in Parkinson's disease, schizophrenia, temperature regulation, pain, and cancer cell growth. Previously, Dr. Grisshammer and his colleagues showed how neurotensin binds to the part of its receptor located on a cell's surface. In this study, they demonstrated how binding changes the structure of the rest of the receptor, which passes through a cell's membrane and into its interior. There neurotensin receptors activate G proteins, a group of molecules inside cells that controls a series of chemical chain reactions.
For these experiments, scientists shot X-rays at crystallized neurotensin receptor molecules. Making crystals of receptors that activate G proteins is difficult. In most studies, scientists have investigated inactive receptors.
“The receptor we crystallized is very close to the active form found in nature,” said Dr. Grisshammer. “We may have the first picture of a peptide-binding G protein-coupled receptor just before it engages with the G protein.”
To achieve their results, the scientists made multiple genetic modifications to a less active version of the neurotensin receptor they had used before. Experiments performed in test tubes showed that mixing the receptor with neurotensin sparked the G protein reactions for which the scientists were looking.
When the scientists looked at the structure of the new crystals, they discovered how binding of neurotensin to the receptor caused critical parts of the receptor located below a cell's surface to change shape. In particular, they saw that a region in the middle of the receptor dropped like a draw bridge to link the neurotensin binding site to parts of the receptor found inside cells that are important for G protein activation. The scientists concluded that this change may prepare the receptor for activating G proteins.
“For years scientists have made educated guesses about how peptide receptors work. Now we may finally know,” said Dr. Grisshammer.
His lab plans to continue its work in order to fully understand how neurotensin and other G protein-coupled receptors translate messages delivered by neuropeptides into reactions inside cells.
###
This work was funded by the NINDS Division of Intramural Research.
References: Krumm et al. “Structural prerequisites for G-protein activation by the neurotensin receptor,” Nature Communications, July 24, 2015. DOI: 10.1038/NCOMMS8895
For more information, please visit: neuroscience.nih.gov/ninds/Home.aspx
The NINDS is the nation's leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit http://www.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



