… in development of psychiatric drugs
Glycine can stimulate or inhibit neurons in the brain, thereby controlling complex functions. Unraveling the three-dimensional structure of the glycine transporter, researchers have now come a big step closer to understanding the regulation of glycine in the brain. These results, which have been published in Nature, open up opportunities to find effective drugs that inhibit GlyT1 function, with major implications for the treatment of schizophrenia and other mental disorders.
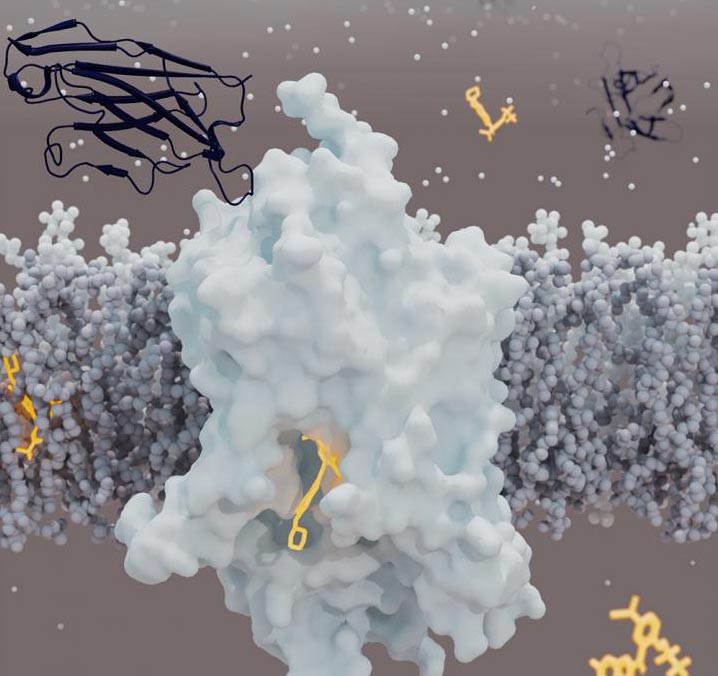
Glycine is the smallest amino acid and a building block of proteins, and also a critical neurotransmitter that can both stimulate or inhibit neurons in the brain and thereby control complex brain functions. Termination of a glycine signal is mediated by glycine transporters that reuptake and clear glycine from the synapses between neurons. Glycine transporter GlyT1 is the main regulator of neurotransmitter glycine levels in the brain, and also important for e.g. blood cells, where glycine is required for the synthesis of heme.
The N-methyl-D-aspartate (NMDA) receptor is activated by glycine, and its poor performance is implicated in schizophrenia. Over the past twenty years, many pharmaceutical companies and academic research laboratories therefore have focused on influencing glycinergic signaling and glycine reuptake delay as a way of activating the NMDA receptor in search of a cure for schizophrenia and other psychiatric disorders. Indeed, several potent and selective GlyT1 inhibitors achieve antipsychotic and pro-cognitive effects alleviating many symptoms of schizophrenia, and have advanced into clinical trials. However, a successful drug candidate has yet to emerge, and GlyT1 inhibition in blood cells is a concern for side effects. Structural insight into the binding of inhibitors to GlyT1 would provide insight in finding new strategies in drug design.
To gain better knowledge about the three-dimensional structure and inhibition mechanisms of the GlyT1 transporter, researchers from the companies Roche and Linkster, and from the European Molecular Biology Laboratory (EMBL) Hamburg, University of Zurich and Aarhus University, have therefore collaborated on investigating one of the most advanced GlyT1 inhibitors. Using a synthetic single-domain antibody (Linkster therapeutics’sybody®) for GlyT1, the research team managed to grow microcrystals of the inhibited GlyT1 complex. By employing a Serial Synchrotron Crystallography (SSX) approach, the team lead by Assistant Professor Azadeh Shahsavar and Professor Poul Nissen from the Department of Molecular Biology and Genetics/DANDRITE, Aarhus University, determined the structure of human GlyT1 using X-ray diffraction data from hundreds of microcrystals. The SSX method is particularly suitable as a method fornew, powerful X-ray sources and opens up for new approaches to, among other things, the development of drugs for various purposes.
The structure is reported in the leading scientific journal Nature and also unveils a new mechanism of inhibition in neurotransmitter transporters in general. Mechanisms have previously been uncovered for, for example, inhibition of the serotonin transporter (which has many similarities to GlyT1) with antidepressant drugs, but it is a quite different inhibition mechanism now found for GlyT1. It provides background knowledge for the further development of small molecules and antibodies as selective inhibitors targeted at GlyT1 and possibly also for new ideas for the development of inhibitors of other neurotransmitter carriers that can be used to treat other mental disorders. Azadeh Shahsavar’s team continues the studies of GlyT1 and will be investigating further aspects of its function and inhibition and the effect of GlyT1 inhibitors in the body.
###
The studies have received financial support from the Novo Nordisk Foundation, the Lundbeck Foundation, the EU-Marie Sklodowska Curie Cofund (EMBL EIPOD postdoc fellowship) and Roche.
The results have been published in the international journal Nature:
Structural insights into the inhibition of glycine reuptake
Azadeh Shahsavar, Peter Stohler, Gleb Bourenkov, Iwan Zimmermann, Martin Siegrist, Wolfgang Guba, Emmanuel Pinard, Steffen Sinning, Markus A. Seeger, Thomas R. Schneider, Roger J. P. Dawson & Poul Nissen
https://doi.org/10.1038/s41586-021-03274-z
For further information, please contact
Assistant Professor Azadeh Shahsavar – ash@mbg.au.dk
Professor Poul Nissen – pn@mbg.au.dk – +45 28992295
Danish Research Institute of Translational Neuroscience–DANDRITE
Nordic EMBL Partnership for Molecular Medicine
Department of Molecular Biology and Genetics
Aarhus University, Denmark