Flexible Quantum Sieve Filters the Fuel of Starship Enterprise
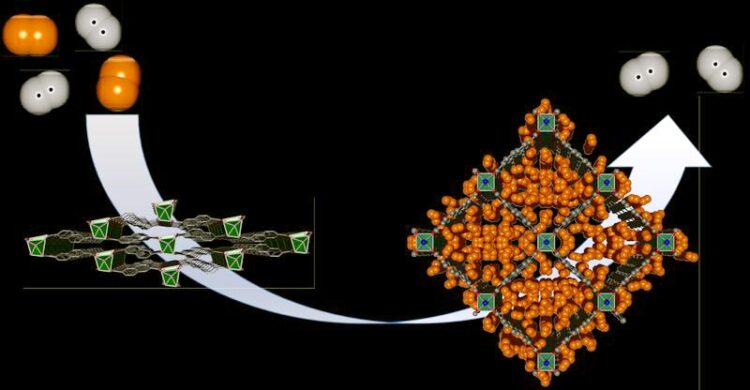
Nur Deuterium kann die Poren des metallorganischen Gerüsts „DUT-8“ öffnen, während Wasserstoff das Gerüst geschlossen lässt. Diese hochselektive Erkennung führt zu einer hohen Trennschärfe bei gleichzeitig hoher Deuterium-Aufnahme.
(c) Dr. Volodymyr Bon
Deuterium, the heavy brother of hydrogen, is considered a promising material of the future – because of its wide range of applications: in science, for energy generation, or in the production of pharmaceuticals. However, the extraction of deuterium from its natural isotope mixture has so far been complex and expensive. With a porous material developed at the Technische Universität Dresden, this could soon be done more efficiently and cost-effectively. The new method has now been published in the scientific journal “Science Advances”.
Starship Enterprise flew through the galaxy using deuterium as fuel. Even if this was science fiction from the 1960s and 70s, research on the real application of the hydrogen isotope for energy generation is still going on today. The main challenge here is the extraction of the isotope. Deuterium (chem. abbrev. D, “heavy” hydrogen) is one of the three natural isotopes of hydrogen, along with protium (H, “normal” hydrogen) and tritium (T, “superheavy” hydrogen). Both deuterium and protium are stable isotopes of hydrogen. Ordinary water and heavy water made from deuterium are similarly stable. Tritium (T) is extremely promising from a technical standpoint, but is not without safety concerns due to its radioactivity.
Deuterium is extracted from heavy water, i.e. water containing deuterium, which is contained to 0.15 per mille in the natural water resources of our earth. To do this, the heavy water is first isolated using chemical and physical processes and then deuterium gas is produced. These processes are so complex and energy-intensive that one gram of deuterium is more expensive than a gram of gold, even though its natural occurrence is many times higher.
But the demand for pure deuterium continues to grow, because its unique physical properties mean that its potential applications are far from exhausted: when used in medicines, deuterium has already been shown to have a life-prolonging effect, albeit initially only for the active ingredient itself. Drugs containing deuterium can be dosed lower, so that their side effects are also reduced. In nuclear reactors, deuterium plays an important role as a moderator. In addition, a mixture of deuterium and tritium or 3Helium is planned to be used as fuel in future fusion reactors. Other fields of application include medicine, life sciences, analysis, and novel TV displays.
In an interdisciplinary collaboration, the groups of Prof. Stefan Kaskel and Prof. Thomas Heine from TU Dresden, together with Dr. Michael Hirscher from the MPI for Intelligent Systems Stuttgart, have now developed a novel separation mechanism for the hydrogen isotopes based on the flexible metal-organic framework “DUT-8” developed at TU Dresden. “Our material enables separation of gaseous deuterium from hydrogen. The unique metal-organic framework DUT-8 is highly flexible and can dynamically adapt its pore size. But this structural response was found to be highly selective: Only deuterium can open the pores while hydrogen leaves the framework closed. This highly selective recognition leads to a high separation selectivity combined with high deuterium uptake ,” explains Stefan Kaskel, professor of Inorganic Chemistry at TU Dresden. With his group, he specializes in novel nanostructured and porous functional materials for energy storage and conversion and has already developed several patented materials.
His material DUT-8, published in 2012, initially showed no hydrogen uptake, neither at high pressure nor at very low temperatures. “During our measurements at the MPI in Stuttgart, we observed for the first time an opening of the structure of DUT-8 under deuterium atmosphere at very low temperatures. Subsequently, we also succeeded in separating hydrogen isotope mixtures experimentally, with the material acting as a kind of flexible and therefore extremely efficient “quantum sieve”,” explains Dr. Michael Hirscher, who has been researching efficient separation mechanisms for hydrogen isotopes at the MPI for Intelligent Systems for several years.
First-principles calculations in conjunction with statistical thermodynamics predict the isotope-selective opening and rationalize them with pronounced nuclear quantum effects. However, there are other so-called isotopologues (molecules of the same elements but different isotopes) of hydrogen, namely HD, HT, DT and T2, which have to be considered in the separation, and those containing T are radioactive. In the group of Thomas Heine, Chair of Theoretical Chemistry at TU Dresden, the behavior of these isotopologues has been simulated. “In this joint work, we have succeeded in replacing safety-related problematic experiments with radioactive material with validated computer simulations and thus in making predictions for potential applications of this isotope-dependent opening effect of DUT-8,” Professor Heine explains. His simulations show that DUT-8 opens only for isotopologues without light H isotopes. For HD, these predictions have already been confirmed experimentally by Dr. Hirscher’s group.
Wissenschaftliche Ansprechpartner:
Prof. Thomas Heine
Theoretical Chemistry
TU Dresden
Email: thomas.heine@tu-dresden.de
Dr. Michael Hirscher
MPI for Intelligent Systems Stuttgart
Email: hirscher@is.mpg.de
Originalpublikation:
Linda Bondorf, Jhonatan Luiz Fiorio, Volodymyr Bon, Linda Zhang, Mariia Maliuta, Sebastian Ehrling, Irena Senkovska, Jack D. Evans, Jan-Ole Joswig, Stefan Kaskel, Thomas Heine, Michael Hirscher. Isotope-selective pore opening in a flexible metal-organic framework. Science Advances. DOI: 10.1126/sciadv.abn7035
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



