Getting to the core of nuclear speckles
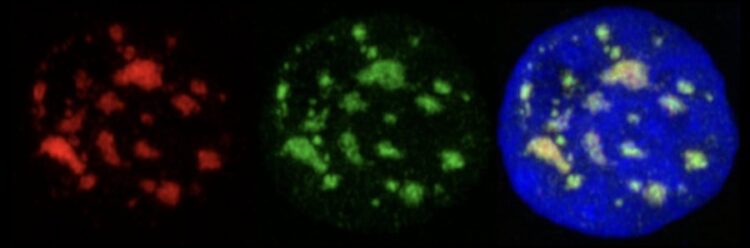
Human cell nucleus under the microscope: Markers for SON (red; left image) and SRRM2/SC35 (green; center image) highlight where nuclear speckles are located (right image) in relation to the cell’s DNA (blue). The nucleus is around 10 micrometers in diameter, which is a hundredth of a millimeter.
Ibrahim Ilik, MPI f. molek. Genetik
Scaffold of sub-cellular structures identified after a hundred years.
Nuclear speckles are tiny agglomerations of proteins in the nucleus of the cell that are involved in the processing of genetic information. Berlin researchers have now discovered how nuclear speckles are constructed and were able to close a long-standing gap in research.
When the famous Spanish physician Santiago Ramón y Cajal looked through his microscope in 1910, he discovered irregular and “transparent lumps” that appeared throughout the nucleus of a neuron. What these nuclear speckles are all about is still largely unclear, even though the biological and medical sciences have experienced several revolutions since then.
“Even though we know quite a bit about their function, we didn’t know how nuclear speckles originate, i.e. what their core consists of,” says Tuğçe Aktaş from the Max Planck Institute for Molecular Genetics.
A Berlin team of scientists led by the Max Planck Research Group leader now identified the molecules that form the scaffold of nuclear speckles and described their results in the scientific journal eLife.The two proteins in question are SON and SRRM2, which are present in different variations throughout the entire animal kingdom. Both molecules are involved in the processing of RNA, which is produced when genes are transcribed. Without these proteins, the speckles dissolve.
Unlike other cell structures, speckles do not have a membrane envelope. They consist of an aggregation of molecules that can dynamically dissolve and reassemble, exhibiting the properties of solids as well as those of liquids. These “condensates” can be found throughout the cell. “Each cellular condensate has a protein that represents its nucleus – in the case of nuclear speckles, there are two,” says Aktaş.
Of red herrings and finding the right path
It is no coincidence that past attempts to identify the lowest common denominator of the mysterious structures have not been successful. “For 30 years scientists have been staining nuclear speckles with a reagent that they did not know very well”, says Aktaş. “We did not realize that we have been in the dark for decades.”
Since the early nineties, nuclear speckles have been visualised with a substance called SC35, which is an antibody that specifically attaches to certain sites in the speckles and can stain them with the help of pigments. Until recently, however, it was assumed that the antibody only recognizes the small protein SRSF2 – an assumption that now turned out to be wrong.
“We wanted to use the antibody as a bait to fish for speckles in the cell,” says İbrahim Avşar Ilık, the lead author of the study. “It was a great surprise to find the protein SRRM2, which was not the intended prey for our experiment.” It turned out that the antibody not only adheres to the already known SRSF2, but especially and particularly well to SRRM2.
Quest in the evolutionary family tree
While the sequence of SRRM2 varies widely in different animal species, the protein has a small section that has been preserved over hundreds of millions of years of evolution. Looking for similar proteins in the evolutionary family tree, the researchers also noticed the protein SON, which was considered by other research groups as a possible critical component of the speckles, too. “We had the idea that the combination of the two proteins could be the fundamental building block of the speckles”, says Ilık.
To test their hypothesis, the team grew human cells with the genes for either SRRM2 or SON switched off. This resulted in only spherical remnants of the speckles in the cell’s nuclei. Once the researchers knocked down both proteins simultaneously, all the speckles dissolved completely and associated proteins were found to be distributed throughout the cell nucleus. “We concluded that SRRM2 and SON must be the scaffold for nuclear speckles,” says Ilık. “Next, we will investigate how the two proteins bind to other molecules and how this process is controlled”.
Historical misinterpretation has consequences
But the results have even more, and perhaps far-reaching consequences. “Now that it is clear that SC35 binds to a different protein than assumed, previous research results on nuclear speckles must be carefully reevaluated,” says Aktaş.
The antibody SC35 has also been widely used in disease research, since the speckles have been implicated in several neurodegenerative conditions such as Huntington’s disease, spinocerebellar ataxia and dentatorubro-pallidoluysis atrophy. “There may be entirely new perspectives for research into these diseases,” says Aktaş.
Wissenschaftliche Ansprechpartner:
Dr. Tuğçe Aktaş
Max Planck Institute for Molecular Genetics
aktas@molgen.mpg.de
+49 30 8413-1550
Originalpublikation:
Ilik İA, Malszycki M, Lübke AK, Schade C, Meierhofer D, Aktaş T. SON and SRRM2 are essential for nuclear speckle formation. Elife 2020;9:e60579. DOI: 10.7554/eLife.60579
https://elifesciences.org/articles/60579
https://www.molgen.mpg.de/4337366/news_publication_16079733_transferred?c=2168
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



