How cells keep their nucleus clean
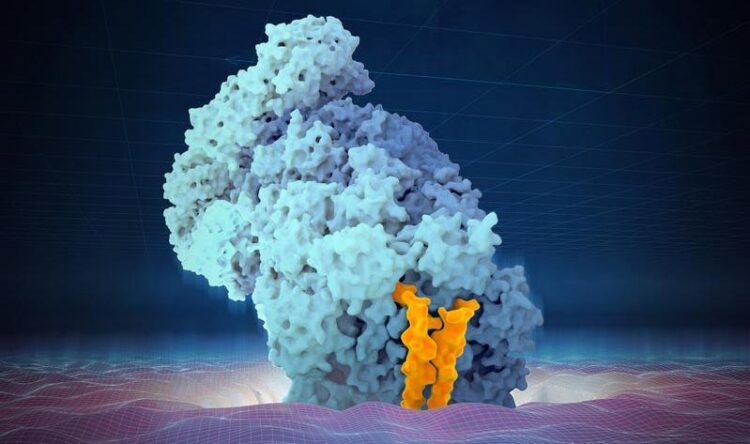
Artist’s interpretation of AKIRIN2’s role in the import of the proteasome into the nucleus. The two ‘fingers’ of AKIRIN2 (in orange) carry the proteasome (light blue) into the nucleus.
(c) IMP
… a fundamental discovery.
Scientists at the Research Institute of Molecular Pathology (IMP) in Vienna have developed a CRISPR-Cas9 screening assay that allows to systematically pinpoint regulators of any gene of interest, including cancer-related genes. Using this approach, they discovered how cells transport their clean-up machinery, the proteasome, into the nucleus to maintain protein balance and get rid of unwanted nuclear proteins. The results of this study are now reported in the journal Nature.
At the Research Institute of Molecular Pathology (IMP) in Austria, scientists in the lab of Johannes Zuber are on the hunt. They are searching for cellular switches that could turn off genes associated with cancer. One of their targets is the gene MYC and its associated protein, which is overly expressed in tumour cells and underlies boosts of cell division.
In a healthy cell, MYC is one of the most short-lived proteins: as soon as its work is done, it needs to be destroyed, or it may cause unwanted cell proliferation. Such is the function of the proteasome, a complex of proteins that acts as the clean-up machinery of the cell. Once a protein is no longer used, the proteasome breaks it apart into smaller peptide pieces.
“The healthy functioning of our organs, tissues, and cells relies on the rapid production and turnover of tens of thousands of proteins. Each protein is controlled by ‘gas pedals’ and ‘brake pedals’, and both can be exploited for the development of drugs,” explains Johannes Zuber, senior scientist at the IMP. “The goal of our study was to pinpoint the gas and brake pedals of cancer-related genes such as MYC, and we found a regulator that we hadn’t expected.”
A surprising finding
Two PhD students in Zuber’s lab devised a CRISPR-Cas9 screening assay that allows to eliminate, or ‘knock out’ any gene in the genome in a time-controlled manner, and to see if it affects the abundance of MYC. Should a knocked-out gene result in a decrease in MYC, it could represent a potential drug target for cancer.
Because MYC is essential for cancer cells to grow, its regulation is challenging to study. “Deleting essential genes leads to very quick cell death. However, our screening method allows us to initiate the gene knockout at any chosen moment, so that we can study the immediate consequences of this knockout before the cells start dying,” explains Melanie de Almeida, co-first author of the study and a Vienna BioCenter PhD student.
With their innovative method, the team was able to get a comprehensive view of the regulators of MYC. One of these regulators was a protein they had never heard of.
“In every screen we ran, we found a small protein called AKIRIN2 that appeared to be essential for switching off MYC. Without AKIRIN2, cells showed a strong increase in MYC levels, which was very similar to what we saw when we disrupted the proteasome.” says Matthias Hinterndorfer, co-first author and Vienna BioCenter PhD student. “It was like the first piece of a puzzle that we wanted to solve.”
AKIRIN2: two fingers pulling the proteasome into the nucleus
The scientists found that AKIRIN2 was consistently associated with the proteasome and not only required for the regulation of MYC, but of many more short-lived proteins. To their surprise, they found that AKIRIN2 only affected nuclear proteins, which accumulated freely in its absence. Thus, they hypothesised that AKIRIN2 might help the proteasome to enter the nucleus to break down unwanted nuclear proteins.
To test their idea, the team harnessed the IMP’s broad expertise in molecular biology and teamed up with David Haselbach, group leader at the IMP and expert in proteasome biology.
“With our biochemical analyses, we characterised the structure of AKIRIN2, and confirmed that it binds to the proteasome: it forms a dimer that looks a bit like two fingers, which attach to the proteasome and carry it from the cytoplasm into the nucleus,” explains Haselbach. “This process is particularly important after each cell division, where AKIRIN2 quickly imports proteasomes from the cytoplasm into the newly formed nuclei of daughter cells.”
“Our work highlights the power of interdisciplinary research and the collaborative spirit at the IMP. We have this unique setting that brings together researchers from different fields of molecular biology, who then build on each other’s expertise to tackle scientific problems from different angles,” says Zuber.
Exploiting AKIRIN2 to develop selective proteasome-inhibiting drugs
Inhibitors that block the activity of the proteasome are already applied to treat various types of cancer. However, existing drugs affect proteasomes in all the cells of our body, which can lead to unwanted side-effects in healthy cells.
“Our study shows that AKIRIN2 is required for bringing proteasomes into the nucleus after each cell division. Drugs blocking the interaction between AKIRIN2 and the proteasome could therefore provide a strategy to selectively inhibit nuclear proteasomes in rapidly dividing cells, such as cancer cells,” says Matthias Hinterndorfer.
To develop such selective proteasome inhibitors, scientists need to better understand how AKIRIN2 functions and how it is regulated. Luckily, the assay that the team pioneered in this study is applicable to virtually any gene – and now can be used to uncover the factors that regulate AKIRIN2.
“Our paper represents a fundamental discovery for biology, but it’s also a major technical advancement for the study of gene regulatory networks,” says Melanie de Almeida. “Our assay may allow scientists to study essential regulators of any protein of interest, including proteins that are related to cancer.”
The ground-breaking study, now published in the journal Nature, combines the development of a novel assay to study essential regulatory pathways in the cell, the discovery of an essential regulator of nuclear proteins, and the characterisation of its 3-dimensional structure.
Wissenschaftliche Ansprechpartner:
johannes.zuber@imp.ac.at
Originalpublikation:
Melanie de Almeida*, Matthias Hinterndorfer*, Hanna Brunner, Irina Grishkovskaya, Kashish Singh, Alexander Schleiffer, Julian Jude, Sumit Deswal, Robert Kalis, Milica Vunjak, Thomas Lendl, Richard Imre, Elisabeth Roitinger, Tobias Neumann, Susanne Kandolf, Michael Schutzbier, Karl Mechtler, Gijs Versteeg, David Haselbach, Johannes Zuber. AKIRIN2 controls the nuclear import of proteasomes in vertebrates. Nature, 2021. DOI: 10.1038/s41586-021-04043-8.
Weitere Informationen:
https://www.nature.com/articles/s41586-021-04043-8
https://www.imp.ac.at/groups/johannes-zuber/
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Parallel Paths: Understanding Malaria Resistance in Chimpanzees and Humans
The closest relatives of humans adapt genetically to habitats and infections Survival of the Fittest: Genetic Adaptations Uncovered in Chimpanzees Görlitz, 10.01.2025. Chimpanzees have genetic adaptations that help them survive…

You are What You Eat—Stanford Study Links Fiber to Anti-Cancer Gene Modulation
The Fiber Gap: A Growing Concern in American Diets Fiber is well known to be an important part of a healthy diet, yet less than 10% of Americans eat the minimum recommended…

Trust Your Gut—RNA-Protein Discovery for Better Immunity
HIRI researchers uncover control mechanisms of polysaccharide utilization in Bacteroides thetaiotaomicron. Researchers at the Helmholtz Institute for RNA-based Infection Research (HIRI) and the Julius-Maximilians-Universität (JMU) in Würzburg have identified a…



