How Proteins Find Their Place in the Cell
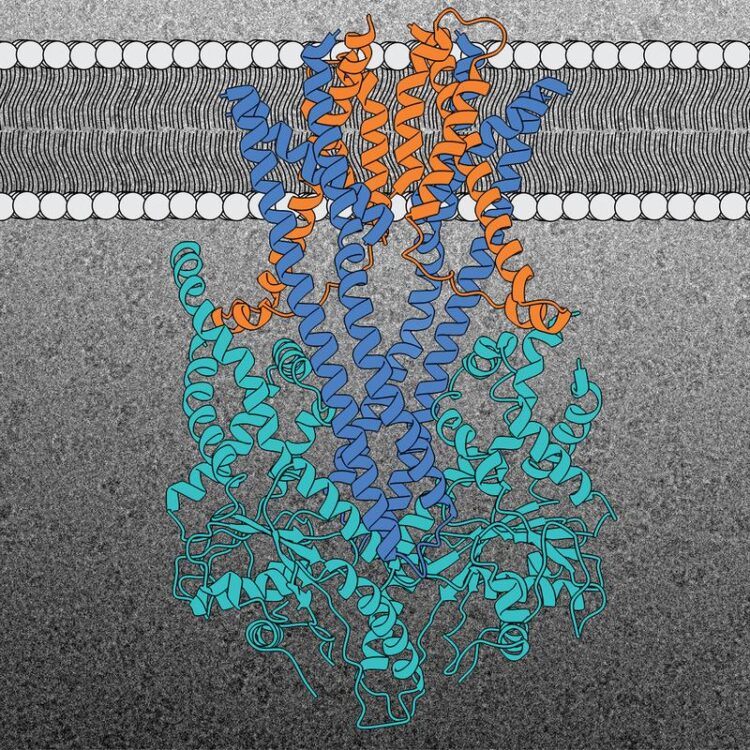
Structure of the GET insertion machine (Get1 in blue, Get2 in orange and Get3 in light blue). A representative cryo-EM image of the complex is shown in the background.
McDowell and Sinning (2020)
Correctly Delivered and Integrated
Heidelberg researchers determine the three-dimensional architecture of a molecular machine that inserts essential proteins into biomembranes
Over a quarter of all proteins in a cell are found in the membrane, where they perform vital functions. To fulfil these roles, membrane proteins must be reliably transported from their site of production in the cell to their destination and correctly inserted into the target membrane. Researchers from the Heidelberg University Biochemistry Center (BZH) have succeeded in determining the three-dimensional structure of a molecular machine responsible for the correct placement of an important membrane protein family – the so-called “tail-anchored” membrane proteins, or TA proteins for short.
An adult human consists of an estimated 100 billion cells. Each one contains countless proteins, the architects and players in life that perform a broad range of functions. A major portion of the proteins in a cell are membrane proteins, i.e. components of the fine membranes (from the Latin membrana) that envelop every cell as well as its small organs, the organelles. Membrane proteins can form channels or pores and perform fundamental tasks such as transport of substances and signal transmission. Therefore, the correct insertion of a membrane protein is crucial for it to fulfil its biological role and, in turn, for the proper function of the cell. But what ensures that the protein ends up at the right membrane and is integrated at the right spot?
Specific signal sequences, small sections of proteins that act like “post codes”, are vital for delivery to the correct location and proper insertion into the membrane. They are detected by molecular sorting machines that deliver the protein to its destination. In some proteins, the signal sequence is found at the end of the molecule, known to scientists as “tail-anchored” or TA membrane proteins. This vital membrane protein family is involved in many cellular processes, including membrane fusion and apoptosis, or programmed cell death.
BZH researchers led by Prof. Dr Irmgard Sinning recently determined the three-dimensional structure of the molecular machine that inserts the TA proteins into the membrane of the endoplasmic reticulum (ER) – an important distribution network inside the cell that is connected to all other organelles. For their structural analyses, the BZH scientists used cryo-electron microscopy (cryo-EM), a method recognised by the Nobel Prize for Chemistry in 2017. “This type of high-resolution structural information is essential to understand the final steps of the protein insertion process into the ER membrane,” explains Prof. Sinning, who directs a research group at the BZH.
The GET insertion machine is responsible for the correct insertion of TA proteins into the ER membrane. GET stands for “guided entry of tail-anchored membrane proteins”. This insertion machine, which has barely changed over the course of evolution from yeast to man, consists of three protein building blocks. Two are located in the ER membrane where they form a kind of cavity (Get1 and Get2). The third one (Get3) is located outside the membrane, acting as the TA protein deliverer. All three components of the GET insertion machine are essential for the correct insertion of the TA protein into the target membrane. Get2 takes the protein from the deliverer and essentially “pushes” it towards the cavity in the interior of the membrane. The Heidelberg researchers uncovered this unexpected detail concerning the interaction between Get2 and Get3 during their analysis of the protein structure. They also showed that two copies of the insertion machine always work closely together to make the integration process more efficient. “The GET insertion machine provides the TA proteins with an energetically favourable route into the membrane,” states Prof. Sinning.
“Small membrane proteins like those found in the GET insertion machine are a challenge for structural biology, so our research required innovative ideas,” adds structural biologist Dr Melanie McDowell. Only in recent years have technical improvements in cryo-EM allowed structures of increasingly smaller protein complexes to be identified in ever greater detail. Heidelberg University therefore established a cryo-EM network (HDcryoNet), making the structural analysis of small membrane protein complexes like the GET insertion machine possible. Prof. Sinning and Dr McDowell believe that their new data provide a crucial missing puzzle piece required to complete the picture of protein transport in the cell and protein insertion into membranes.
The study, with participating scientists from the University of Oxford (UK) and the University Medical Center Göttingen, was funded mainly by the German Research Foundation (DFG). The research results were published in the journal “Molecular Cell”.
Contact:
Heidelberg University
Communications and Marketing
Press Office, phone +49 6221 54-2311
presse@rektorat.uni-heidelberg.de
Wissenschaftliche Ansprechpartner:
Prof. Dr Irmgard Sinning and Dr Melanie McDowell
Heidelberg University Biochemistry Center
Phone +49 6221 54-4781 and 54-4788
irmi.sinning@bzh.uni-heidelberg.de
melanie.mcdowell@bzh.uni-heidelberg.de
Originalpublikation:
M.A. McDowell, M. Heimes, F. Fiorentino, S. Mehmood, Á. Farkas, J. Coy-Vergara, D. Wu, V. Schmid, R. Heinze, J. R. Bolla, K. Wild, D. Flemming, S. Pfeffer, B. Schwappach, C.V. Robinson, & I. Sinning: Structural basis of tail-anchored membrane protein biogenesis by the GET insertase complex. Molecular Cell (2020) 80: 72-86.e7, doi: 10.1016/j.molcel.2020.08.012
Weitere Informationen:
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



