How the coronavirus defends itself against our immune system
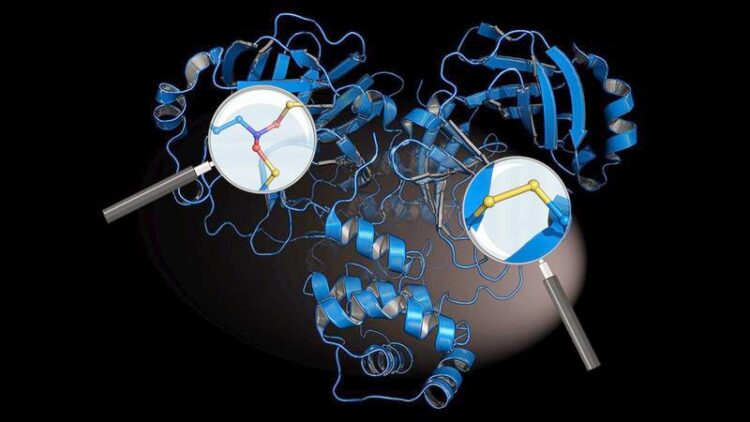
Atomic structure of the main protease of the coronavirus SARS-CoV-2 showing the newly discovered chemical protection switches SONOS (magnifying glass on the left) and disulphide (magnifying glass on the right)
Credit: Kai Tittmann
Research team identifies “protective switches” in the protein of the SARS-CoV-2 virus.
Over 700 million people were infected and almost seven million died, making SARS-CoV-2 the most devastating pandemic of the 21st century. Vaccines and medication against Covid-19 have been able to mitigate the course of the disease in many people and contain the pandemic. However, the danger of further outbreaks has not been averted. The virus is constantly mutating, which enables it to infect human cells and multiply more and more effectively. In addition, it is developing a variety of strategies against the human immune system in a “molecular arms race”. A team led by researchers from the University of Göttingen has now discovered various “protective switches” in the coronavirus that shield it from attacks by the immune system. The results were published in Nature Communications.
The researchers identified two previously unknown chemical protective switches in the virus’s main “protease” – a crucial protein of the coronavirus. The most important drug against Covid-19, called Paxlovid®, targets this protein. The virus uses its main protease to cut out the other virus proteins in our infected cells, thus driving its own replication. It uses the amino acid cysteine to do this. “From a chemical point of view, this could be an Achilles heel for the coronavirus, as cysteines can be destroyed by highly reactive oxygen radicals, which our immune system uses to fight viruses,” explains Professor Kai Tittmann, Molecular Enzymology Research Group at Göttingen University, who led and coordinated the study.
The protective switches mean the virus’s main protease is protected against the immune system’s bombardment by oxygen radicals: the protein is stabilised by one cysteine forming a disulphide with an adjacent cysteine via two sulphur atoms. This prevents the cysteine from being destroyed. At the same time, a bridge known as SONOS connects three parts of the protein between sulphur atoms (S), oxygen atoms (O), and a nitrogen atom (N). This prevents radicals from damaging its three-dimensional structure. Tittmann says: “It is fascinating to see how chemically elegant and effective the coronavirus is in defending itself against the immune system. Interestingly, a coronavirus discovered earlier – severe acute respiratory syndrome, also known as SARS-CoV-1 – which triggered the 2002 to 2004 outbreak, also has these protective switches. This is the first time this has been shown.”
Despite this scientific first, the researchers were not satisfied with just discovering “protective switches”. With the chemical blueprint to hand, they set about searching for molecules that can bind precisely to the “protective switches”, therefore inhibiting the virus’s main protease. They identified such molecules not only in the test tube, but also in infected cells. “This type of molecule opens up the potential for new therapeutic interventions which will stop coronaviruses in their tracks,” says Lisa-Marie Funk, first author of the study, also at Göttingen University’s Molecular Enzymology research group.
The study was made possible by funding from the Covid-19 Research Network Lower Saxony (COFONI) and the German Research Foundation (DFG). This interdisciplinary study involved researchers from the Faculty of Biology and Psychology, and the Faculty of Chemistry at the University of Göttingen, the University Medical Center Göttingen (UMG), the Max Planck Institute for Multidisciplinary Sciences, the Hannover Medical School and the Universities of Düsseldorf, Hamburg and Lübeck.
Original publication: Funk et al. Multiple redox switches of the SARS-CoV-2 main protease in vitro provide opportunities for drug design. Nature Communications (2024). DOI: 10.1038/s41467-023-44621-0
Contact:
Professor Kai Tittmann
University of Göttingen
Göttingen Center for Molecular Biosciences (GZMB)
Molecular Enzymology Research Group
Julia Lermontowa Weg 3, 37077 Göttingen, Germany
Tel: +49 (0)551 39-177811
Email: ktittma@gwdg.de
www.uni-goettingen.de/en/89703.html
Journal: Nature Communications
DOI: 10.1038/s41467-023-44621-0
Method of Research: Experimental study
Subject of Research: Cells
Article Title: Multiple redox switches of the SARS-CoV-2 main protease in vitro provide opportunities for drug design.
Article Publication Date: 9-Jan-2024
Media Contact
Melissa Sollich
University of Göttingen
melissa.sollich@uni-goettingen.de
Office: 49-551-392-6228
Original Source
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



