Identify, engage, assassinate: How seahorse-like toxins kill insects
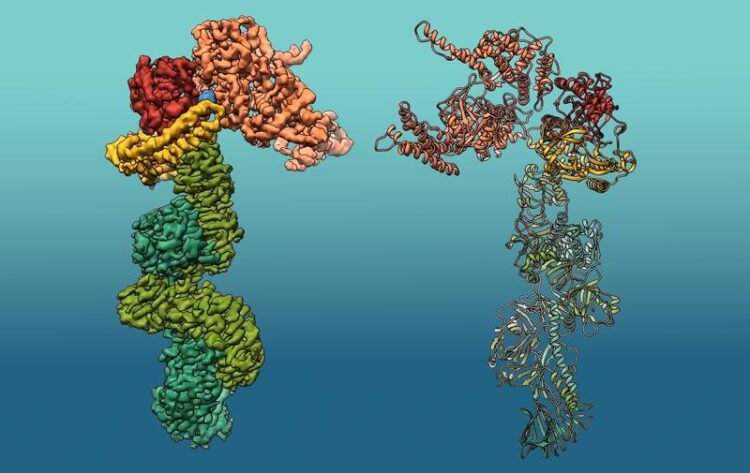
3D structure of ‘Makes caterpillars floppy 1’ (Mcf1). The structure of Mcf1 resembles a seahorse with a head containing several toxic payloads, while the tail region can attach to target cells.
(c) MPI of Molecular Physiology
Max Planck researchers from Dortmund reveal the first-ever detailed structure of the bacterial toxin Mcf1.
Insect-killing bacteria typically release toxins to slay their hosts. The bacterium Photorhabdus luminescens, for example, pumps insect larvae full of the lethal ‘Makes caterpillars floppy 1’ (Mcf1) toxin, leading them to first become droopy and then dead. However, it has so far been a mystery how Mcf1 unfolds its devastating effect. Researchers led by Stefan Raunser, Director at the Max Planck Institute of Molecular Physiology in Dortmund, successfully used cryo-electron microscopy (cryo-EM) and biochemical assays to characterize the first-ever Mcf1 structure, allowing them to propose a molecular mechanism of the toxin’s action. Understanding how bacterial toxins perform their deadly task in such detail is very useful for engineering novel biopesticides, thereby reducing the use of barely specific chemical agents with harmful side effects for the ecosystem.
“Ours is the first-ever structural study of this toxin,” explains Alexander Belyy, first author of the study. The challenge of the project, which took a decade of work, lay in the fact that the protein is relatively large and composed of multiple modules, each devoted to a specific function. “The use of our cutting-edge cryo-EM equipment and computational power was instrumental to resolve this structure,” says Stefan Raunser.

Mechanism of action of Mcf1 toxin. After binding of the toxin to the surface of the host cell, the head region of the toxin is endocytosed. Released toxic payloads impair essential physiological pathways ultimately leading to cell death. (c) MPI of Molecular Physiology
Cryo-EM allows researchers to obtain 3D images of a protein at near-atomic resolution, in this case at 3.6 Ångstrom – meaning that details 200,000 times smaller than the width of a human hair could be observed. The scientists were able to show that the structure of Mcf1 resembles a seahorse with a head containing several toxic payloads, while the tail region can attach to target cells. After the toxin is released by bacteria in the host insect, three domains in its tail region identify and bind to the target cell’s membrane. Another domain of the tail then transfers the head across the membrane into the cell’s cytoplasm. Once inside, the head interacts with local proteins to stimulate the release of two toxic payloads. These deadly modules disrupt the activity of essential proteins in the cell, leading to its death – and ultimately to the death of the entire insect within 24 hours of intoxication.
New organic pest control agents
Intriguingly, the researchers found that the modular structure of the tail and the initial steps of Mcf1 intoxication are highly similar to toxins from Clostridioides difficile, a human pathogen responsible for more than 120,000 hospitalizations in Europe annually. “Our study, which was originally aimed at improving biopesticides, will also have an impact on the understanding of human diseases,” adds Philipp Heilen, co-first author of the study.
Looking forward, the MPI scientists want to elucidate in molecular detail how the toxic payloads of Mcf1 lead to cell death. “This new knowledge also enables engineering of highly specific insecticidal toxins,” says Stefan Raunser hinting at another future direction of research: creating new toxin variants useful for ecological pest control.
Originalpublikation:
Belyy A, Heilen P, Hagel P, Hofnagel O, Raunser S (2023). Structure and activation mechanism of the Makes caterpillars floppy 1 toxin. Nat Commun.
Doi: 10.1038/s41564-023-01571-z.
Weitere Informationen:
https://www.mpi-dortmund.mpg.de/news/bacterial-toxin-seahorse-insect
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



