Important milestone on the way to transition metal catalysis with aluminum
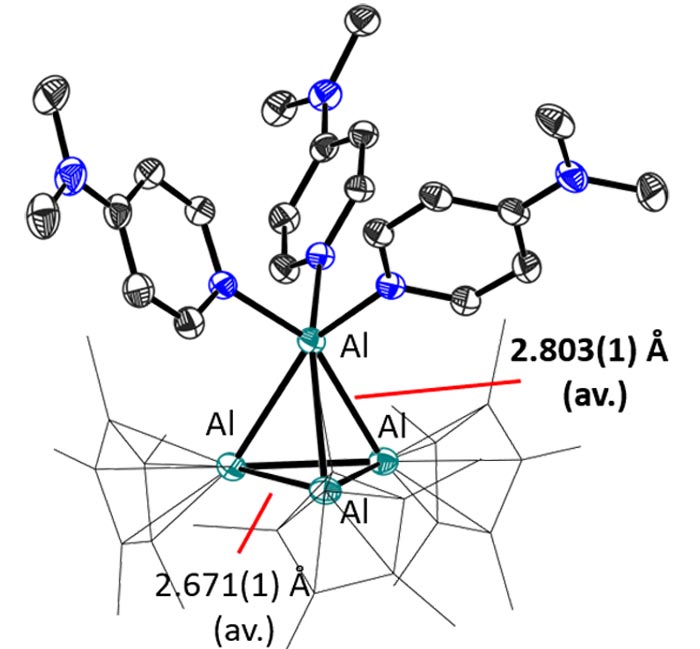
Addition of ligands, such as dimethylaminopyridine in this case, breaks the dimeric structure of the cation and the lone aluminum atom shows its strong acceptor character. Image: provided by the research group
Chemists successfully synthesize a cationic, low-valent aluminum complex salt via metathesis.
The chemists Philipp Dabringhaus, Julie Willrett and Prof. Dr. Ingo Krossing from the Institute of Inorganic and Analytical Chemistry at the University of Freiburg have succeeded in synthesizing the low-valent cationic aluminum complex [Al(AlCp*)3]+ by a metathesis reaction. The team presents their research work in the journal Nature Chemistry.
“In chemistry, cationic low-valent aluminum compounds are highly sought after due to their potential transition metal-like ambiphilic reactivity. However, numerous previous attempts to synthesize cationic low-valent aluminum compounds by oxidative or reductive methods have been largely unsuccessful,” Krossing explains. So far, he said, there has been only one example of a cationic, low-valent aluminum compound, but it cannot be prepared by rational synthesis. “We now show that there is an unexpectedly easy access to low-valent aluminum complexes with metathesis after all,” Krossing says. In metathesis, partial structures are simply exchanged between the reaction partners.
Aluminum as a cheaper alternative for catalysis
The Freiburg chemists prepared the salt [Al(AlCp*)3]+[Al(OC{CF3)3}4]– from the Schnöckel tetramer (AlCp*)4, in which aluminum is already present in the +1 oxidation state. The (AlCp*)4 reacted with Li[Al{OC(CF3)3}4] and the reaction mixture immediately turned from yellow to red. When the reaction mixture was crystallized, the scientists obtained the [Al(AlCp*)3]+[Al(OC{CF3)3}4]–-salt as dark purple crystals. “X-ray crystallographic, UV spectrometric and computational studies indicate the presence of the dimeric structure both in the solid state and in solution at high concentration and low temperature, but at low concentration and room temperature the monomer forms. This clearly indicates ambiphilic reactivity of the cation,” Dabringhaus said.
“Consequently, this salt can potentially be used as a building block for an [:Al(L)3]+-salt that, due to its cationic nature, might be able to perform reversible oxidative additions and reductive eliminations of small molecules,” Krossing explains. “This brings us one step closer to our long-term goal of achieving catalysis – currently done with expensive and rare transition metals – with aluminum. Aluminum is the second most abundant element in the Earth’s crust and capable of doing so in principle, as our work shows. But unfortunately, it will probably be at least another 20 years before our research on this is applied.”
“Behind the Paper” article on the Nature Chemistry website
Fact Overview:
- Prof. Dr. Ingo Krossing heads the Chair of Molecular and Coordination Chemistry at the Institute of Inorganic and Analytical Chemistry at the University of Freiburg and is a member of the Living, Adaptive and Energy-autonomous Materials Systems (livMatS) Cluster of Excellence.
- Krossing has received an Advanced Grant from the European Research Council (ERC) for his project “InnoChem – Innocent Deelectronation Chemistry,” in which he is conducting research on a universally valid redox scale.
- In 2018, Krossing was admitted to the Heidelberg Academy of Sciences and Humanities, and in 2020 to the Leopoldina National Academy of Sciences.
- Krossing’s research focuses on: weakly coordinating anions, reactive cations, catalysis for energy conversion, unified acidity and redox scales, and battery electrolytes and materials. More on Ingo Krossing’s research.
- Original publication: Dabringhaus, P., Willrett, J., Krossing, I. (2022): Synthesis of a low-valent Al4+ cluster cation salt. Nature Chemistry. DOI: 10.1038/s41557-022-01000-4
Contact:
Prof. Dr. Ingo Krossing
Institute for Inorganic and Analytical Chemistry
University of Freiburg
Phone: +49 (0)761/203-6122
E-Mail: ingo.krossing@ac.uni-freiburg.de
Franziska Becker
Office of University and Science Communications
University of Freiburg
Phone: +49 (0)761/203-54271
E-Mail: franziska.becker@zv.uni-freiburg.de
Journal: Nature Chemistry
DOI: 10.1038/s41557-022-01000-4
Media Contact
Rimma Gerenstein
University of Freiburg
info@pr.uni-freiburg.de
Office: 761-203-4302
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



