Inner workings of an essential protein trafficking complex
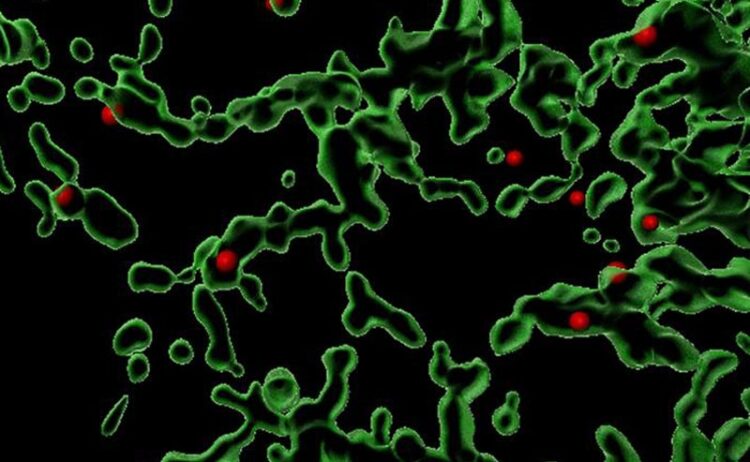
This image is a 3D reconstruction showing sites (in red) where the Coat Protein Complex II (COPII) is facilitates the packaging of various proteins within a mammalian cell. The green areas are the endoplasmic reticulum, where protein sorting and trafficking takes place.
Image courtesy of Anjon Audhya
Like mail carriers who manage to deliver their parcels through snow, rain, heat and gloom, a critical group of mammalian proteins helps cells function properly even under less-than-ideal conditions.
Using state-of-the-art cell imaging and genome editing technology, University of Wisconsin–Madison scientists have begun to unravel how this collection of proteins performs its essential service. The discovery could eventually help researchers better understand and develop new treatments for diseases like cancer, diabetes and those that cause immune dysfunction.
Led by Anjon Audhya, a professor in the Department of Biomolecular Chemistry, the research team sought to better understand how the Coat Protein Complex II, or COPII, functions. COPII is an enormously important group of proteins responsible for transporting roughly a third of all proteins that function in mammalian cells.
COPII was a subject of the 2013 Nobel Prize in Physiology or Medicine, which was awarded to a trio of scientists for their work defining how proteins are sorted and transported around cells. This new research builds on some of those discoveries.
There are millions of proteins inside mammalian cells, and they perform a wide variety of duties. Cells must ensure that proteins are moved efficiently to their proper places, so they can perform their cellular roles — an intricate task requiring precision. Previous research identified COPII as an essential part of this process, but no one had recorded exactly how this set of proteins goes about packaging and transporting other proteins around cells.
To do so, Audhya and his colleagues used the CRISPR/Cas9 genome editing tool to add a tag, which could be chemically linked to a bright, fluorescent dye, to individual proteins involved in controlling the traffic flow within cells, including some that make up the COPII complex. With the tag, the scientists could follow the proteins as they moved about living cells.
Using a technique called lattice light-sheet imaging, the team tracked how COPII helps get cellular proteins, including molecules destined for other places, where they’re supposed to go — something that had never been done before.
The team described their advance in a paper recently published in the journal Nature Communications. Audhya described it in terms of the postal system. Researchers knew that COPII functions like postal workers who pick up and deliver parcels, but they had never tracked these workers as they sorted packages through the cell’s distribution and delivery systems.
“We can now see that envelope in the mailbox, see how the mail carrier comes to the mailbox to pick up the letter and then drive away,” says Audhya, who is the senior associate dean for basic research, biotechnology and graduate studies at the School of Medicine and Public Health.
The researchers discovered that, on average, this delivery process takes between 45 and 60 seconds under normal conditions. However, when cells receive subpar nutrition, as they sometimes do thanks to certain diseases and environmental conditions, this process slows down significantly, at least until cells can adapt over time.
Through a series of experiments, Audhya and his colleagues were able to identify a single protein named Sec23 that was capable of helping restore COPII’s trafficking system after disruption. When the scientists increased how much Sec23 was produced inside cells, they saw a change in the rate in which cells transported proteins, “something we never anticipated when we started this work,” Audhya says. “Sec23 seems to be the central player in regulating the function of the COPII complex.”
Identifying what triggers Sec23 to promote COPII function has potential implications for a number of diseases. For instance, cancer cells often grow prodigiously in nutrient-starved environments, in part by producing more of certain proteins that promote growth. Understanding the molecular mechanisms that underlie this property could identify new targets for therapies.
Beyond that, a more precise picture of the process by which cells correctly prepare and deliver proteins can help inform our basic understanding of proper cell function and what can go awry in diseases such as cancer, Type 2 diabetes, neurodegenerative conditions and immune disorders.
“Understanding these fundamental processes and the regulatory systems that exist in cells can ultimately pave the way to developing more rational approaches to disease intervention,” says Audhya.
This work was supported in part by National Institutes of Health grants GM134865 and GM008688, as well as shared resources available in the UWCCC Flow Cytometry Laboratory and the UW Optical Imaging Core.
–Will Cushman, wcushman@wisc.edu, 608-263-1986
Journal: Nature Communications
Method of Research: Experimental study
Subject of Research: Cells
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



