Key chemical intermediates in pollutant-to-fuel reaction identified
Abstract
Credit: University of Tsukuba
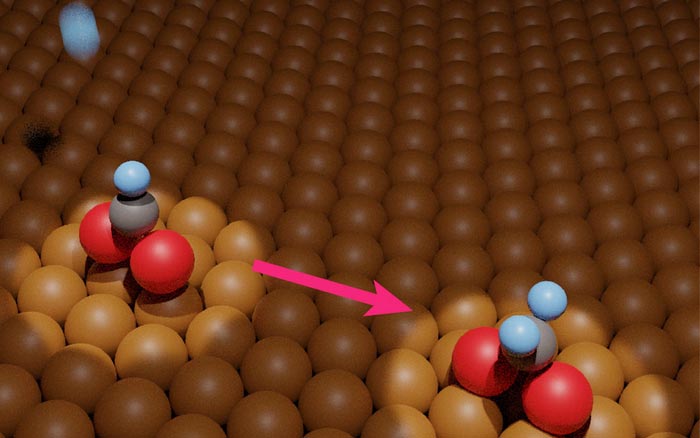
Caught in the act:
Researchers from the University of Tsukuba and collaborating partners experimentally measured hydrogenation of metal-adsorbed formate. This development will facilitate practical conversion of carbon dioxide pollutant into methanol fuel.
Carbon dioxide pollution continues to change the global climate. Researchers know how to pinpoint such pollution, even on a regional and near-real-time basis, as highlighted this year in Science Advances (April 22). As part of a solution to carbon dioxide pollution, many studies focus on how to convert this pollutant into a fuel, such as methanol. Copper-based catalysts are a tool for such conversions. Understanding the corresponding step-by-step chemistry is essential for optimizing conversion of carbon dioxide pollutant into methanol fuel. However, the details of this chemistry remain unclear; experiments are needed to test hypotheses that are currently based on computer simulations.
Now, in a study recently published in Journal of the American Chemical Society researchers from the University of Tsukuba and collaborating partners have experimentally measured hydrogenation of copper-adsorbed formate. This study will help researchers optimize critical steps in the aforementioned pollutant-to-fuel process, and thus accelerate methanol production.
“Hydrogenation of carbon dioxide into methanol is a potential key technology for producing fuel and chemical feedstocks, but optimizing the reaction remains difficult,” explains Dr. Kotaro Takeyasu, senior author. “That’s because it’s difficult to experimentally detect chemical intermediates in the step-by-step reaction mechanism.”
Infrared reflection absorption spectroscopy and temperature-programmed desorption were critical to obtaining two main findings. First, at a temperature of 200 Kelvin, exposure to atomic hydrogen corresponded to hydrogenation of adsorbed formate. The exact chemical nature of the product isn’t yet clear. It was also found that, at a temperature of 250 Kelvin, the hydrogenated formate decomposed back into adsorbed formate or gaseous formaldehyde, in a 96:4 ratio.
“On the basis of our experimental and computational work, the activation energy of the hydrogenation of adsorbed formate is approximately 121 kilojoules per mole,” states Dr. Takeyasu. “Our results are consistent with reported results of methanol synthesis studies.”
Copper-zinc alloys are particularly common in this line of work. The research group is currently investigating how the activation energies reported in the present study compare with particularly useful catalytic alloys, which also require experimental and computational investigations.
The results of this study will help researchers optimize methanol production from carbon dioxide. Such work will help convert an atmospheric pollutant into fuel for vehicles, and chemical feedstocks for industry. It provides a means of adding value to carbon dioxide, which is commonly considered to be waste. By optimizing the hydrogenation reaction described here, researchers might have a new tool for making maximum use of limited resources.
This work was partly supported by Grants in Aid for Scientific Research for Challenging Research (grant No. JP20K21099), for Transformative Research Areas (A) “Hyper-Ordered Structure Science” (grant No. JP20H05883), and for Innovative Area “Hydrogenomics” (grant No. JP18H05519) from the Japan Society for the Promotion of Science.
Original Paper
The article, “Hydrogenation of formate species using atomic hydrogen on a Cu(111) model catalyst,” was published in Journal of the American Chemical Society at DOI: 10.1021/jacs.2c02797
Correspondence
Assistant Professor TAKEYASU Kotaro
Faculty of Pure and Applied Sciences, University of Tsukuba
Related Link
Faculty of Pure and Applied Sciences
Journal: Journal of the American Chemical Society
DOI: 10.1021/jacs.2c02797
Article Title: Hydrogenation of formate species using atomic hydrogen on a Cu(111) model catalyst
Article Publication Date: 28-Jun-2022
Media Contact
YAMASHINA Naoko
University of Tsukuba
kohositu@un.tsukuba.ac.jp
Original Source
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



