The Inner Life of a Giant – Max Planck Researchers Gain New Insights into Protein Degradation
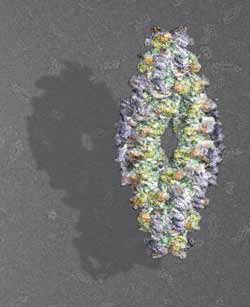
3D-model of the active human TPPII-complex<br>Graphic: Beate Rockel/Copyright: MPI of Biochemistry<br>
Scientists of the Max Planck Institute of Biochemistry (MPIB) in Martinsried near Munich, Germany, have now uncovered the structure and the operating mechanism of an important component of the human cellular degradation machinery, tripeptidyl peptidase II (TPPII).
“Decoding the structure of TPPII is a crucial milestone towards understanding the complex activation and control of protein degradation”, says Beate Rockel, scientist at the MPIB. The results of the study have now been published in the journal Structure.
Proteins, the molecular building blocks and machines of the cell, are composed of long chains of amino acids. When such a chain has to be degraded, it is first unfolded and then cleaved into shorter pieces, so-called peptides. Tripeptidyl peptidase II (TPPII), which was analyzed by scientists in the department of MPIB director Wolfgang Baumeister, is one of the factors that take over further degradation. It chops the peptides into even smaller bits which, after some additional steps, can be recycled for the assembly of new proteins. TPPII is a large complex consisting of 32 to 40 identical subunits, which are inactive on their own. The complex becomes functional, when the subunits join into two strands twisted around each other. The complex is approximately 100 times larger than most other protein-degrading enzymes. “TPPII is a real giant amongst cellular proteins”, says PhD student Anne-Marie Schönegge. “Solving the structure of such a colossus is a difficult task.”
Bit by Bit towards the Complete Structure
The researchers of the MPIB combined different methods of structural biology and models to determine the structure and operating mechanism of TPPII in detail. In collaboration with scientists from the Lawrence Berkeley National Laboratory in Berkeley, they had successfully solved the atomic structure of TPPII-subunits of the fruit fly by X-ray-crystallography. In a subsequent step, this structure served as the basis to calculate a model of human TPPII-subunits.
Using cryoelectron microscopy and single-particle reconstruction, the scientists were able to determine the structure of complete and active TPPII-complexes of the fruit fly and humans – but only at medium resolution. By combining the structure of the complete complexes with the more detailed atomic models of individual subunits, the co-workers of the research department “Molecular Structural Biology” could now solve the detailed structural organization of human TPPII: the subunits enclose a cavity system which traverses the whole TPPII complex and harbors the catalytic sites.
By fitting the structures of the inactive subunits into the structure of the active complex, the scientists pinpointed regions that are supposed to undergo changes upon activation of TPPII. These regions include the active site and the entrances into the cavity-system inside the complex. Beate Rockel also hopes for other benefits out of this work: “Insights into the TPPII structure could contribute to the development of new drugs in the future, since there are indications that TPPII may be involved in diseases such as muscle wasting, adiposis and cancer.” [VS]
Original publications
Schönegge, A., Villa, E., Hegerl, R., Peters, J., Förster, F., Baumeister, W. and Rockel, B.: The structure of human tripeptidyl peptidase II as determined by a hybrid approach. Structure 20(4): 593–603, April 4, 2012
DOI: 10.1016/j.str.2012.01.025
Chuang, C. K., Rockel, B., Seyit, G., Walian, P. J., Schönegge, A., Peters, J., Zwart, P. H., Baumeister, W. and Jap, B. K.: Hybrid molecular structure of the giant protease tripeptidyl peptidase II. Nat Struct Mol Biol 17(8): 990-996, August 1, 2010
DOI: 10.1038/nsmb.1870
Contact
Prof. Dr. Wolfgang Baumeister
Molecular Structural Biology
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
E-Mail: baumeist@biochem.mpg.de
http://www.biochem.mpg.de/baumeister
Dr. Beate Rockel
Molecular Structural Biology
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
E-Mail: rockel@biochem.mpg.de
Anja Konschak
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Phone: +49 (0) 89 8578-2824
E-Mail: konschak@biochem.mpg.de
http://www.biochem.mpg.de
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Largest magnetic anisotropy of a molecule measured at BESSY II
At the Berlin synchrotron radiation source BESSY II, the largest magnetic anisotropy of a single molecule ever measured experimentally has been determined. The larger this anisotropy is, the better a…

Breaking boundaries: Researchers isolate quantum coherence in classical light systems
LSU quantum researchers uncover hidden quantum behaviors within classical light, which could make quantum technologies robust. Understanding the boundary between classical and quantum physics has long been a central question…

MRI-first strategy for prostate cancer detection proves to be safe
Active monitoring is a sufficiently safe option when prostate MRI findings are negative. There are several strategies for the early detection of prostate cancer. The first step is often a…



