Making and breaking of chemical bonds in single “nanoconfined” molecules
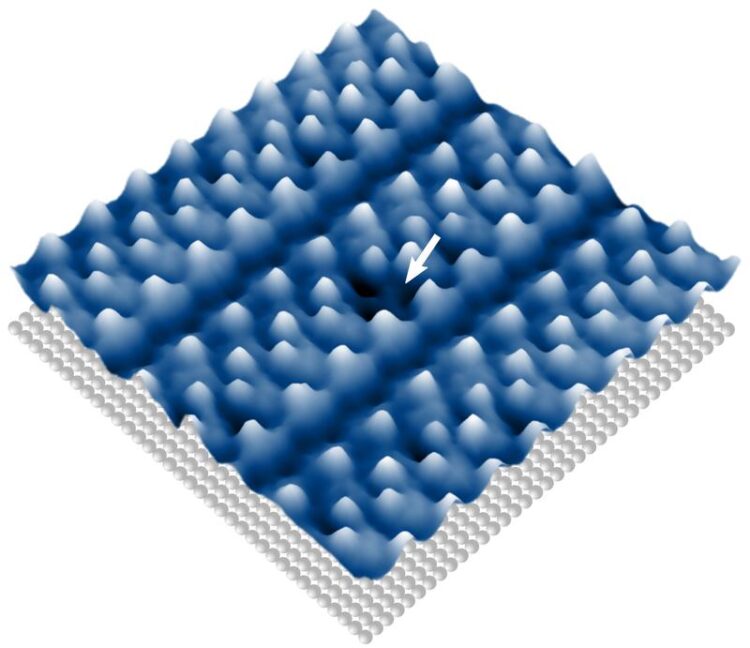
Image showing how the catalytically active molecules arranged themselves into almost perfect single-layer structures on a polished silver surface.
Credit: Ole Bunjes
Research team investigates reactivity of single molecules under controlled microscopic conditions.
Researchers around the world are working to develop efficient materials to convert CO2 into usable chemical substances – work that is particularly pressing in view of global warming. A team from the University of Göttingen, Germany, and the Ulsan National Institute for Science, South Korea, has discovered a new and promising approach: catalytically active molecules are nanoconfined – meaning they are put into an environment that leaves very little space for the single molecules – on a surface that serves as a conductive electron supplier. These molecules promote specific chemical reactions. Such hybrid systems make use of both the properties of the molecules and the properties of the substrate. The results were published in Science Advances.
The first step for the team was to deposit the catalytically active molecules as a vapour onto polished silver before examining them with a high-resolution scanning tunneling microscope built in Göttingen. “To our absolute astonishment, the molecules arrange themselves, as if by magic, into almost perfect single-layer structures on the surface,” says Lucas Paul, PhD student, University of Göttingen, and co-author of the study.
“In addition to imaging individual molecules, the energy of the injected electrons can be adjusted so precisely in the scanning tunneling microscope that chemical reactions can be induced and observed in a single molecule,” explains physicist Professor Martin Wenderoth. Wenderoth led the project together with chemist Professor Inke Siewert, at the University of Göttingen’s Collaborative Research Centre 1073 ‘Atomic Scale Control of Energy Conversion’. Siewert adds, “We are able to very precisely break individual chemical bonds.”
The researchers show that molecules that are particularly densely packed on the surface have altered chemical properties. Thus, exclusively for the “trapped” molecules the bond can be broken and subsequently also restored, since the separated part of the molecule can only move very slightly away from the rest of the molecule. “This shows how a lack of space, at an atomic level, can be used to manipulate chemical reactions,” says first author Ole Bunjes, University of Göttingen.
The research team wants their experiments to contribute to the development of efficient molecular surface systems with precisely determined properties. In addition, they want to investigate whether their new system is suitable as a molecular data memory.
Original publication: Bunjes et al, “Making and breaking of chemical bonds in single nanoconfined molecules”, Science Advances 2022: DOI: 10.1126/sciadv.abq7776
Contact:
Professor Martin Wenderoth
University of Göttingen
Faculty of Physics
Friedrich-Hund-Platz 1, 37077 Göttingen, Germany
Tel: +49 (0)551 39-29367
Email: martin.wenderoth@uni-goettingen.de
Journal: Science Advances
DOI: 10.1126/sciadv.abq7776
Method of Research: Experimental study
Subject of Research: Not applicable
Article Title: Making and breaking of chemical bonds in single nanoconfined molecules
Article Publication Date: 9-Sep-2022
Media Contact
Melissa Sollich
University of Göttingen
melissa.sollich@uni-goettingen.de
Office: 49-551-392-6228
Original Source
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



