Mechanism of an enzyme for biofuel production
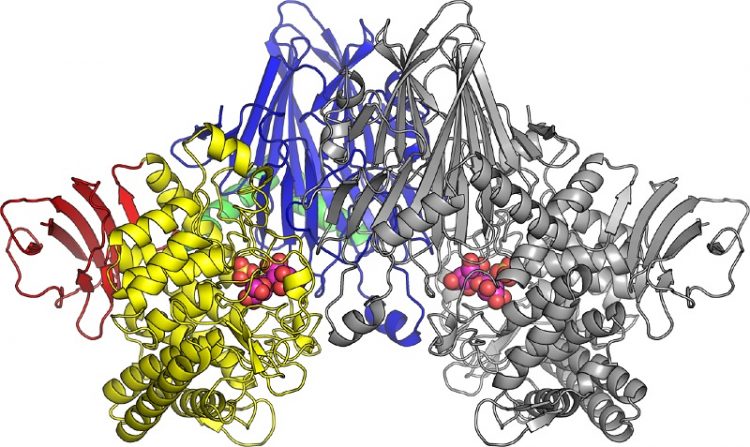
*The three-dimensional structure of cellobionic acid phopshorylase. © 2015 Shinya Fushinobu.
A University of Tokyo research group has revealed for the first time the three-dimensional structure and mechanism of action of a key enzyme of bio-fuel production, cellobionic acid phosphorylase (CBAP). This result is important basic information for developing the technology to make bio-fuel and other chemical products from biomass.
It has been long thought that hydrolytic enzymes (cellulases) were the main contributors to microbial degradation of cellulose. Recently, the existence of oxidative cellulose-degrading enzymes that dramatically increase the activity efficiency of cellulases have been noted.
When these enzymes degrade cellulose, cellobionic acid is produced. However, it was completely unknown how the cellulolytic microbes further metabolize this compound.
In 2013, one of the members of the research group, Associate Professor Hiroyuki Nakai at the Graduate School of Science and Technology, Niigata University, discovered a new enzyme, cellobionic acid phosphorylase (CBAP).
CBAP catalyzes the degradation of cellobionic acid to produce compounds that are prone to further metabolism and fermentation. Therefore, this enzyme is a missing link between the oxidative cellulose degradation and bioethanol fermentation pathways in microorganisms. However, the three dimensional structure of the enzyme and the mechanism by which it degraded cellobionic acid remained unknown.
In this latest research, the research group lead by Professor Shinya Fushinobu at the University of Tokyo, Graduate School of Agricultural and Life Sciences, used X-ray crystallography to reveal the three-dimensional structure of CBAP isolated from marine bacteria. In addition, the structure of CBAP in complex with cellobionic acid was determined (figure), and the reaction mechanism for decomposing cellobionic acid was revealed.
“This research is extremely interesting from a scientific perspective, but could also contribute to the development of biorefinery technologies that produce biofuels such as ethanol and other useful compounds via biomass degradation by microbes,” says Professor Fushinobu.
*Image
Two cellobionic acid phosphorylases molecules pair up to create a dimer. The colored left half and the gray right half are each one enzyme molecule. Cellobionic acid and sulfuric acid ions (a compound similar to phosphoric acid) bound to cellobionic acid phosphorylase are expressed as spheres in the figure.
Paper
Young-Woo Nam, Takanori Nihira, Takatoshi Arakawa, Yuka Saito, Motomitsu Kitaoka, Hiroyuki Nakai, and Shinya Fushinobu, “Crystal structure and substrate recognition of cellobionic acid phosphorylase playing a key role in oxidative cellulose degradation by microbes”, The Journal of Biological Chemistry Vol. 290, No. 30, pg 18281-18292, doi: 10.1074/jbc.M115.664664.
Associated links
UTokyo Research article
Media Contact
More Information:
http://www.researchsea.comAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

NASA: Mystery of life’s handedness deepens
The mystery of why life uses molecules with specific orientations has deepened with a NASA-funded discovery that RNA — a key molecule thought to have potentially held the instructions for…

What are the effects of historic lithium mining on water quality?
Study reveals low levels of common contaminants but high levels of other elements in waters associated with an abandoned lithium mine. Lithium ore and mining waste from a historic lithium…

Quantum-inspired design boosts efficiency of heat-to-electricity conversion
Rice engineers take unconventional route to improving thermophotovoltaic systems. Researchers at Rice University have found a new way to improve a key element of thermophotovoltaic (TPV) systems, which convert heat…



