Microgravity analog culture profoundly affects microbial infection process in 3-D human tissue models
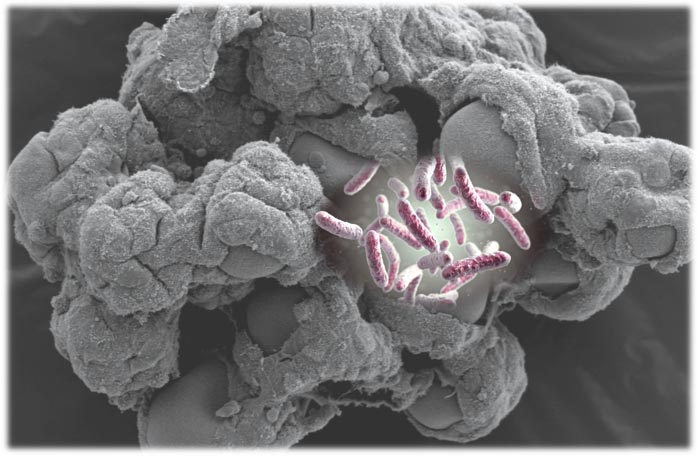
Image of Salmonella bacteria set against an illustration of the 3-D tissue model used in the study.
Credit: Salmonella graphic by drmicrobe
Infectious microbes have evolved sophisticated means to invade host cells, outwit the body’s defenses and cause disease. While researchers have tried to puzzle out the complicated interactions between microorganisms and the host cells they infect, one facet of the disease process has often been overlooked – the physical forces that impact host-pathogen interactions and disease outcomes.
In a new study, corresponding authors Cheryl Nickerson, Jennifer Barrila and their colleagues demonstrate that under low fluid shear force conditions that simulate those found in microgravity culture during spaceflight, the foodborne pathogen Salmonella infects 3-D models of human intestinal tissue at much higher levels, and induces unique alterations in gene expression.
This study advances previous work by the same team showing that physical forces of fluid shear acting on both the pathogen and host can transform the landscape of infection.
Understanding this subtle interplay of host and pathogen during infection is critical to ensuring astronaut health, particularly on extended space missions. Such research also sheds new light on the still largely mysterious processes of infection on earth, as low fluid shear forces are also found in certain tissues in our bodies that pathogens infect, including the intestinal tract.
While the team has extensively characterized the interaction between conventionally grown shake flask cultures of Salmonella Typhimurium and 3-D intestinal models, this study marks the first time that S. Typhimurium has been grown under the low fluid shear conditions of simulated microgravity and then used to infect a 3-D model of human intestinal epithelium co-cultured with macrophage immune cells, key cell types targeted by Salmonella during infection.
The 3-D co-culture intestinal model used in this study more faithfully replicates the structure and behavior of the same tissue within the human body and is more predictive of responses to infection, as compared with conventional laboratory cell cultures.
Results showed dramatic changes in gene expression of 3-D intestinal cells following infection with both wild-type and mutant S. Typhimurium strains grown under simulated microgravity conditions. Many of these changes occurred in genes known to be intimately involved with S. Typhimurium’s prodigious ability to invade and colonize host cells and escape surveillance and destruction by the host’s immune system.
“A major challenge limiting human exploration of space is the lack of a comprehensive understanding of the impact of space travel on crew health,” Nickerson says. “This challenge will negatively impact both deep space exploration by professional astronauts, as well as civilians participating in the rapidly expanding commercial space market in low Earth orbit. Since microbes accompany humans wherever they travel and are essential for controlling the balance between health and disease, understanding the relationship between spaceflight, immune cell function, and microorganisms will be essential to understand infectious disease risk for humans.”
Nickerson, who co-directed the new study with Jennifer Barrila, is a researcher in the Biodesign Center for Fundamental and Applied Microbiomics and is also a professor with ASU’s School of Life Sciences. The research appears in the current issue of the journal Frontiers in Cellular and Infection Microbiology
Life-altering force
Life on earth has diversified into an almost incomprehensibly vast array of forms, evolving under wildly dissimilar environmental conditions. Yet one parameter has remained constant. Throughout the 3.7-billion-year history of life on earth, all living organisms evolved under, and respond to, the pull of Earth’s gravity.
For more than 20 years, Nickerson has been a pioneer in exploring the effects of the reduced microgravity environment of spaceflight on a range of pathogenic microbes and the impact on interactions with human cells and animals they infect. She and her colleagues have doggedly pursued this research in both land-based and spaceflight settings, the results of which helped lay the foundation for the rapidly growing research field, mechanobiology of infectious disease, the study of how physical forces impact infection and disease outcomes.
Among their important findings is that the low fluid shear conditions associated with the reduced gravity environment of spaceflight and spaceflight analog culture are similar to those encountered by pathogens inside the infected host, and that these conditions can induce unique changes in the ability of pathogenic microbes like Salmonella to aggressively infect host cells and exacerbate disease, a property known as virulence.
The infectious agent explored in the new study, Salmonella Typhimurium, is a bacterial pathogen responsible for gastrointestinal disease in humans and animals. Salmonella is the leading cause of death from food-borne illness in the United States. According to the CDC, Salmonella bacteria cause about 1.35 million infections, 26,500 hospitalizations, and 420 deaths in the United States each year. Foods contaminated by the bacteria are the primary source for most of these illnesses.
Salmonella infection typically causes diarrhea, fever, and stomach cramps, beginning 6 hours to 6 days after infection. Illness from the disease usually lasts 4 to 7 days. In severe cases, hospitalization may be required.
Shear probability?
Cells in mammalian organisms, including humans, as well as the bacterial cells that infect them, are exposed to extracellular fluid flowing over their outer surfaces. Just as a gentle downstream current will affect the pebbles in the underlying streambed differently than a raging torrent, so the force of fluid gliding over cell surfaces can cause changes to affected cells. This liquid abrasion of cell surfaces is known as fluid shear.
Since spaceflight experiments are rare and access to the space research platform is currently limited, researchers often simulate the low fluid shear conditions that microbes encounter during culture in spaceflight by growing cells in liquid growth media within a device known as a rotating wall vessel bioreactor or RWV. As the cylindrical reactor rotates, cells are maintained in suspension, gently and continuously tumbling in their surrounding culture medium. This process mimics the low fluid shear conditions of microgravity that cells experience during culture in spaceflight.
The team has also shown that this fluid shear level is relevant to conditions that microbial cells encounter in the human intestine and other tissues during infection, triggering changes in gene expression that can help some pathogens better colonize host cells and evade the immune system’s efforts to destroy them.
Portrait of an intruder
The study found significant changes in both gene expression and ability to infect 3-D intestinal models by Salmonella bacteria cultured in the RWV bioreactor. These experiments involved two S. Typhimurium strains, one unaltered or wild type strain and one mutant strain.
The mutant strain was otherwise identical to the wild type but lacked an important protein known as Hfq, a major stress response regulator in Salmonella. In earlier research, Nickerson and her team discovered that Hfq acts as a master regulator of Salmonella’s infection process in both spaceflight and spaceflight analog culture. They later discovered additional pathogens that also use Hfq to regulate their responses to these same conditions.
Unexpectedly, in the current study, the hfq mutant strain was still able to attach, invade into, and survive within 3-D tissue models at levels comparable to the wild type strain. In agreement with this finding, many genes responsible for Salmonella’s ability to colonize human cells, including those associated with cell adherence, motility, and invasion were still activated in the mutant strain under simulated microgravity conditions, despite the removal of Hfq.
From the host perspective, the 3-D intestinal co-culture model responded to Salmonella infection by upregulating genes involved in inflammation, tissue remodeling, and wound healing at higher levels when the bacteria were grown under simulated microgravity conditions prior to use in infection studies. This was observed for both wild type and hfq mutant strains of the pathogen.
Data from this new spaceflight analog study reinforces previous findings from the team’s 2006, 2008 and 2010 Space Shuttle experiments. In particular, the 2010 flight experiment conducted aboard Space Shuttle Discovery, called STL-IMMUNE, used the same wild type strain of S. Typhimurium to infect a 3-D model of human intestinal tissue made from the same epithelial cells used in the new study.
Several commonalities were observed between host cell responses to infection in the new spaceflight analog study and those previously reported when infections took place in true spaceflight during the STL-IMMUNE experiment. These results further reinforce the RWV as a predictive ground-based spaceflight analogue culture system that mimics key aspects of microbial responses to true spaceflight culture.
“During STL-IMMUNE, we discovered that infection of a human 3-D intestinal epithelial model by Salmonella during spaceflight induced key transcriptional and proteomic biosignatures that were consistent with enhanced infection by the pathogen,” Barrila says. “However, due to the technical challenges of performing in-flight infections, we could not quantify whether the bacteria were actually attaching and invading into the tissue at higher levels. The use of the RWV bioreactor as a spaceflight analog culture system in our current study has been a powerful tool which allowed us to explore this experimental question at a deeper level.”
New horizons
Astronauts face a double risk from infectious disease during their missions far from earth. The combined rigors of spaceflight act to weaken their immune systems. At the same time, some pathogens like Salmonella may be triggered by low fluid shear conditions induced by microgravity to become more effective infectious agents.
With longer spaceflight missions in the advanced planning stages and the advent of civilian space travel rapidly emerging, safeguarding space travelers from infectious disease is vital.
Studies like the current one are also helping to pull back the curtain on the infection process, revealing foundational details with broad relevance for the battle against diseases, on Earth and beyond.
Journal: Frontiers in Cellular and Infection Microbiology
DOI: 10.3389/fcimb.2022.705647
Method of Research: Experimental study
Article Title: Spaceflight analogue culture enhances the host-pathogen interaction between salmonella and a 3-D biomimetic intestinal co-culture model
Article Publication Date: 31-May-2022
Media Contact
Richard Harth
Biodesign Institute at ASU
Richard.Harth@asu.edu
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



