How nerve cells grow
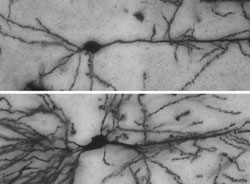
In the brain of mice, which cannot produce Nedd4-1, the extensions of nerve cells are shorter and of much simpler construction (example top) than in the brain of normal mice (example bottom). Image: Hiroshi Kawabe<br>
Brain researcher Hiroshi Kawabe has discovered the workings of a process that had been completely overlooked until now, and that allows nerve cells in the brain to grow and form complex networks. The study, which has now been published in the journal Neuron, shows that an enzyme which usually controls the destruction of protein components has an unexpected function in nerve cells: it controls the structure of the cytoskeleton and thus ensures that nerve cells can form the tree-like extensions that are necessary for signal transmission in the brain. (Neuron, February 11, 2010)
In order to be able to receive signals from other cells, nerve cells form complex extensions called dendrites (from the Greek ‘dendron’ meaning tree). The growth of dendrites in the human brain takes place mainly during late embryonic and infantile brain development. During this phase, dendrites, with a total length of many hundred kilometres, grow from the 100 billion nerve cells in our brain. The result is a highly-complex network of nerve cells that controls all bodily functions – from breathing to complicated learning processes.
In order that this incredible growth phase of brain development does not lead to chaos, the growth of the dendrites must be accurately controlled. In fact, a large number of signal processes control the direction and the speed of dendrite growth by influencing the structure of the cytoskeleton, which is inside the growing dendrite and responsible for its shape and extension.
The Göttingen-based brain researcher Hiroshi Kawabe has now discovered exactly how the growth of the cytoskeleton is controlled during the dendrite development. Using specially bred genetically engineered mice, the Japanese guest scientist, who conducts research at the Max Planck Institute for Experimental Medicine, discovered that the Nedd4-1 enzyme is essential for regular dendrite growth. Nedd4-1 is an enzyme that usually controls the degradation of protein components in cells by combining them with another protein called ubiquitin. The cell identifies these ubiquitinated molecules as “waste” and degrades them. In some cases, however, the ubiquitination does not lead to the degradation of the marked protein but changes its function instead.
Nedd4-1 prevents degradation of the cytoskeleton
Hiroshi Kawabe has now shown that the Nedd4-1 enzyme ubiquitinates a signal protein called Rap2, and thus prevents it causing the dismemberment of the cytoskeleton and the collapse of the dendrites. “As long as Nedd4-1 is active, the nerve cell dendrites can grow normally,” reports Kawabe. “In its absence, the dendrite growth comes to a standstill and previously formed dendrites collapse, with dramatic consequences for the function of nerve cell networks in the brain.” There are, however, probably a number of parallel operating signal paths which control the dendrite growth. This explains why nerve cells can also form dendrites without Nedd4-1 – albeit significantly fewer in number and shorter. The Nedd4/Rap2/TNIK mechanism would then be only one of several that can partially compensate each other.
Kawabe's discovery provides important new insight into the mechanisms which control the development of the brain. “What is surprising is that no-one has investigated this before,” says the Japanese biochemist. Scientists have long been aware that Nedd4-1 is one of the most prevalent ubiquitination enzymes in nerve cells and is produced with great frequency in the developmental phase when nerve cells grow and form their dendrites. As Kawabe points out, the function of Nedd4-1 has already been investigated in dozens of studies. “But very little work has been carried out on its role in nerve cell development, which would have been the obvious thing to do.”
Original work:
Kawabe, H., Neeb, A., Dimova, K., Young, S.M.Jr. Takeda, M., Katsurabayashi, S., Mitkovski, M., Malakhova, O.A., Zhang, D.-E., Umikawa, M., Kariya, K., Goebbels, S., Nave, K.-A., Rosenmund, C., Jahn, O., Rhee, J.-S. and Brose, N.
Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development in cortical neurons.
Neuron 65, 358-372 (2010)
Contact:
Dr. Hiroshi Kawabe
Max-Planck-Institute for Experimental Medicine, Göttingen
Tel.: +49 (0)551 / 3899 720
E-mail: kawabe@em.mpg.de
Media Contact
More Information:
http://www.mpg.de/english/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Pinpointing hydrogen isotopes in titanium hydride nanofilms
Although it is the smallest and lightest atom, hydrogen can have a big impact by infiltrating other materials and affecting their properties, such as superconductivity and metal-insulator-transitions. Now, researchers from…

A new way of entangling light and sound
For a wide variety of emerging quantum technologies, such as secure quantum communications and quantum computing, quantum entanglement is a prerequisite. Scientists at the Max-Planck-Institute for the Science of Light…

Telescope for NASA’s Roman Mission complete, delivered to Goddard
NASA’s Nancy Grace Roman Space Telescope is one giant step closer to unlocking the mysteries of the universe. The mission has now received its final major delivery: the Optical Telescope…



