New test diagnoses Lyme disease within 15 minutes
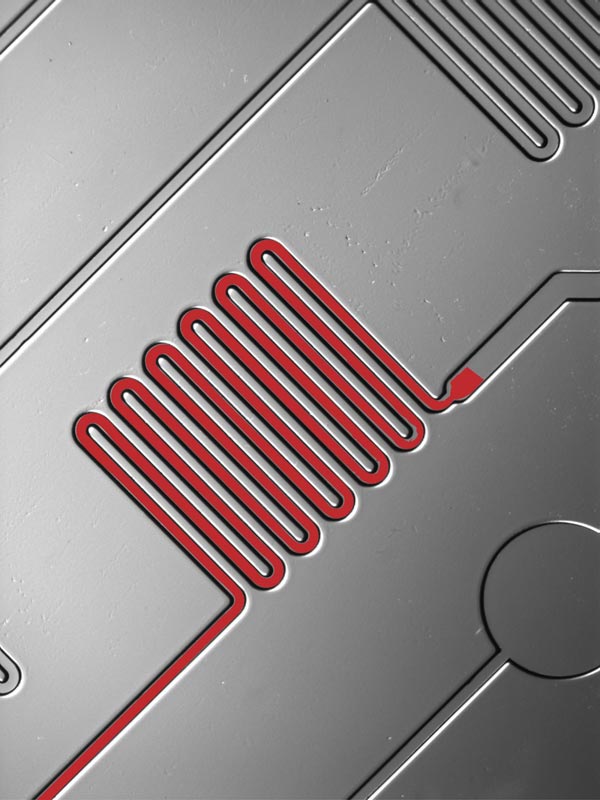
This is a zoomed photo of fluid moving through a small channel in the microfluidic chip. Credit: Sia Lab/Columbia Engineering and OPKO Health
Some 300,000 people in the U.S. are diagnosed with Lyme disease every year. Caused by Borrelia burgdorferi and transmitted by the bite of infected Ixodes ticks, the disease if left untreated can cause serious neurologic, cardiac, and/or rheumatologic complications.
Current testing for Lyme disease, called the standard 2-tiered approach or the STT, involves running two complex assays (ELISA and western blot) to detect antibodies against the bacterium, and requires experienced personnel in a lab, and a few hours to carry out and interpret.
A team led by Sam Sia, professor of biomedical engineering at Columbia Engineering, has developed a rapid microfluidic test that can detect Lyme disease with similar performance as the STT in a much shorter time–15 minutes.
“Our findings are the first to demonstrate that Lyme disease diagnosis can be carried out in a microfluidic format that can provide rapid quantitative results,” says Sia, whose lab is focused on using microfluidics to build low-cost, integrated devices for performing sophisticated medical tests, together with developing new treatment modalities based on cell therapy and implantable devices.
“This means that our test could easily be used directly in a doctor's office, obviating having to send the samples out to a laboratory that needs at least a couple of hours, if not days, to get test results.”
Sia's group worked in collaboration with Maria Gomes-Solecki from Immuno Technologies, which found a combination of three proteins that identified antibodies specific to the B. burgdorferi bacterium in the serum, and OPKO Health, which provided microfluidic cassettes. Their findings were published today in the Journal of Clinical Microbiology.
The researchers evaluated 142 samples, including patients with early Lyme disease, healthy individuals from areas where Lyme disease is endemic, and those with Lyme arthritis. They first screened a set of known diagnostic Lyme disease biomarkers for their ability to detect Lyme disease infection. They then tested the top three biomarkers using a standard enzyme immunoassay, and then mChip-LD, an advanced microfluidic platform developed by Sam Sia, to test the samples.
When tested against additional samples of serum from people with Lyme disease, the multiplexed set of biomarkers was more sensitive than standard Lyme disease tests, while also exhibiting high specificity. The team found that it was better at picking up signs of Lyme disease infection in early-stage samples–possibly because it was able to detect antibodies that peak in the first weeks after someone is infected with Lyme disease.
When the test was run on Sia's mChip-LD platform, it worked very well, showing strong potential for the development of a point-of-care test for Lyme disease. “While the assay will require more refinement and testing before it can be approved for widespread use as a test for Lyme disease, our results are very exciting,” says one of the study's lead authors, Siddarth Arumugam, who is a PhD student in Sia's lab. “It will help so many people if we can develop a single, rapid, multiplexed diagnostic test to identify Lyme disease stage that can be used in doctors' offices.”
Sia is the co-founder of Claros Diagnostics, whose underlying microfluidics technology is now being commercialized by OPKO Health and was recently approved by the FDA for testing for prostate cancer. He and Gomes-Solecki are now planning a more thorough clinical validation study to see whether the performance of the Lyme microfluidic platform holds up.
###
About the Study
The study is titled “A Multiplexed Serologic Test for Diagnosis of Lyme Disease for Point-of-Care Use.”
Authors are: Siddarth Arumugam a; Samiksha Nayak a; Taylor Williams b; Francesco Serra di Santa Maria a; Mariana Soares Guedes b,c; Rodrigo Cotrim Chaves a; Vincent Linder d; Adriana R. Marques e; Elisabeth J. Horn f; Susan J. Wong g; Samuel K. Sia a; and Maria Gomes-Solecki b,c.
a Department of Biomedical Engineering, Columbia Engineering
b Immuno Technologies Inc, Memphis, TN
c Department of Microbiology, Immunology and Biochemistry, University of Tennessee Health Science Center
d OPKO Diagnostics LLC, Woburn, MA
e Lyme Disease Studies Unit, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health
f Lyme Disease Biobank, Portland, OR
g Wadsworth Center, New York State Department of Health, Axelrod Institute
The study was supported by Public Health Service grant (R44 AI096551), and in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Conflicts of interest: M.G.S. is an employee of Immuno Technologies, Inc, and V.L. was an employee of OPKO Diagnostics, LLC while engaged in the research project. We thank the National Institutes of Health, National Institute of Allergy and Infectious Diseases for funding support (grant R44 AI096551) to M.G.S. via Immuno Technologies, Inc. M.G.S. holds 5% or more financial interest in Immuno Technologies, Inc. M.G.S. and A.R.M. hold relevant patents. V.L. declares a financial interest in OPKO Health. S.A., S.N., F. S. S. M., T.W., R.C.C., M.G., S.J.W. and S.K.S. declare no competing financial interests.
###
LINKS:
Paper: https:/
DOI: 10.1128/JCM.01142-19
http://bme.
http://engineering.
https:/
Columbia Engineering
Columbia Engineering, based in New York City, is one of the top engineering schools in the U.S. and one of the oldest in the nation. Also known as The Fu Foundation School of Engineering and Applied Science, the School expands knowledge and advances technology through the pioneering research of its more than 220 faculty, while educating undergraduate and graduate students in a collaborative environment to become leaders informed by a firm foundation in engineering. The School's faculty are at the center of the University's cross-disciplinary research, contributing to the Data Science Institute, Earth Institute, Zuckerman Mind Brain Behavior Institute, Precision Medicine Initiative, and the Columbia Nano Initiative. Guided by its strategic vision, “Columbia Engineering for Humanity,” the School aims to translate ideas into innovations that foster a sustainable, healthy, secure, connected, and creative humanity.
Media Contact
More Information:
http://dx.doi.org/10.1128/JCM.01142-19All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

iENA Silver Medal for Observer of Atoms
In the field of precision engineering and mechatronics systems, novel innovations shape the future of technologies like nano-fabrication technology and high-precision devices. Honoring Excellence: The IMMS Patent Recently, the IMMS…

Biodegradable Plastics from Waste
In a joint research, Bioprocess engineer Prof. Dr.-Ing. Sebastian Riedel from the Berlin University of Applied Sciences (BHT) and Prof. Dr. Jaewook Myung from the Korea Advanced Institute of Science…

NASA successfully integrates Roman mission’s telescope, instruments
NASA’s Nancy Grace Roman Space Telescope team has successfully integrated the mission’s telescope and two instruments onto the instrument carrier, marking the completion of the Roman payload. Now the team…



