Novel treatment strengthens bones in genetic disease
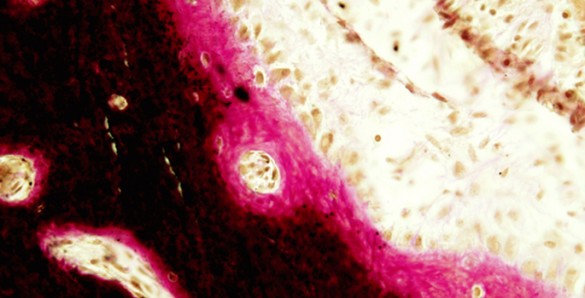
Mice missing the gene neurofibromin in bone cells have more non-calcified bone (osteoid), shown in pink, compared to normal mice. In this image, calcified bone is black and bone-forming osteoblasts lining the deposited osteoid are orange-brown.
The researchers demonstrated in a mouse model of the disorder that the enzyme asfotase-alpha improves bone growth, mineralization and strength. The findings, reported in the journal Nature Medicine, “suggest that we can make bone stronger and better by injecting this drug, and possibly prevent fractures in patients with neurofibromatosis,” said Florent Elefteriou, Ph.D., director of the Vanderbilt Center for Bone Biology.
While he is excited about the results, Elefteriou emphasized the challenge of moving from mouse to human studies. “It’s very difficult to set up a clinical trial in patients with a rare disease; it will have to be an international effort to pool these patients,” he said.
Neurofibromatosis type-1 (NF1) is caused by mutations in the gene for neurofibromin, a protein that regulates cellular signaling pathways. The disorder causes nervous system tumors and skeletal pathologies including scoliosis, bone fragility, fracture and pseudoarthrosis (non-union of the bone following fracture).
Fractures are treated surgically to stabilize the bone and promote healing. Some families opt for amputation, to spare their children the pain of repeated surgeries, Elefteriou said.
“We wondered if there might be a way to prevent the fractures from happening in the first place,” he said.
It was difficult to even propose non-surgical preventive treatments, however, because it was unclear how mutations in neurofibromin cause skeletal pathologies.
To investigate the molecular pathology of NF1, Elefteriou and his colleagues, including first author Jean de la Croix Ndong, Ph.D., have studied a mouse model of the disorder. They noticed in histological stains of bone tissue that the mice had an accumulation of non-mineralized matrix, a condition called hyperosteoidosis.
They have now discovered that hyperosteoidosis in the mice is caused by accumulation of the molecule pyrophosphate, a strong inhibitor of bone mineralization. They found that in the absence of neurofibromin, the expression of certain genes is upregulated. These include genes that enable increased production and transport of pyrophosphate and a gene that prevents calcium and phosphate from depositing on collagen fibers.
In addition, the bone-forming cells fail to differentiate (mature) into “proper tenure-track osteoblasts,” Elefteriou said, which means the cells don’t produce alkaline phosphatase, the enzyme that normally breaks down pyrophosphate.
“That’s a fourth factor preventing mineralization and the formation of new good bone,” he said.
The investigators decided to try clearing the accumulated pyrophosphate by treating the mice with asfotase-alpha, an engineered form of alkaline phosphatase. Asfotase-alpha is currently in clinical trials for hypophosphatasia, another rare genetic disease affecting bone formation.
They found that asfotase-alpha treatment improved bone mass, mineralization and bone mechanical properties in the mouse model of NF1.
“This could be a drug that would prevent fractures and help these kids pass through the early rapid growth period and reach the point where they aren’t as likely to fracture the bone,” Elefteriou said.
To explore whether the molecular pathology of the disease is the same in humans as in the mouse model, the researchers studied pseudoarthrosis tissue biopsies from patients with NF1. They found that the gene that promotes pyrophosphate synthesis is upregulated, suggesting a similar molecular pathology and supporting the notion that asfotase-alpha may be a successful treatment in patients.
There’s much work to be done first, Elefteriou cautions. He notes that although the enzyme therapy corrects the functional defect of pyrophosphate accumulation, it does not correct the failure of osteoblasts to differentiate. This may indicate a need for long-term and combination drug therapy, which the researchers will examine in the mouse model.
They will also continue to collaborate with members of the Children’s Tumor Foundation Bone Consortium to develop clinical trials.
“I think we’ve made great progress in this area,” Elefteriou said. “It’s exciting that instead of fixing the bones after they break, we might have a drug now to prevent the fractures.”
Other Vanderbilt Center for Bone Biology contributors to the studies included Alexander Makowski, Sasidhar Uppuganti, Guillaume Vignaux, Ph.D., Koichiro Ono, Ph.D., Daniel Perrien, Ph.D., and Jeffry Nyman, Ph.D. Additional contributors included Simon Joubert, Ph.D., at Alexion Pharmaceuticals, Serena Baglio, Ph.D., and Donatella Granchi, Ph.D., at Istituto Ortopedico Rizzoli in Bologna, Italy, David Stevenson, M.D., at the University of Utah and Jonathan Rios, Ph.D., at University of Texas Southwestern Medical Center.
This research was supported by a Young Investigator Award from the Children’s Tumor Foundation and by grants from the National Institutes of Health (AR055966, RR027631, TR001105).
Contact:
Leigh MacMillan, (615) 322-4747
leigh.macmillan@vanderbilt.edu
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



