Origami with DNA
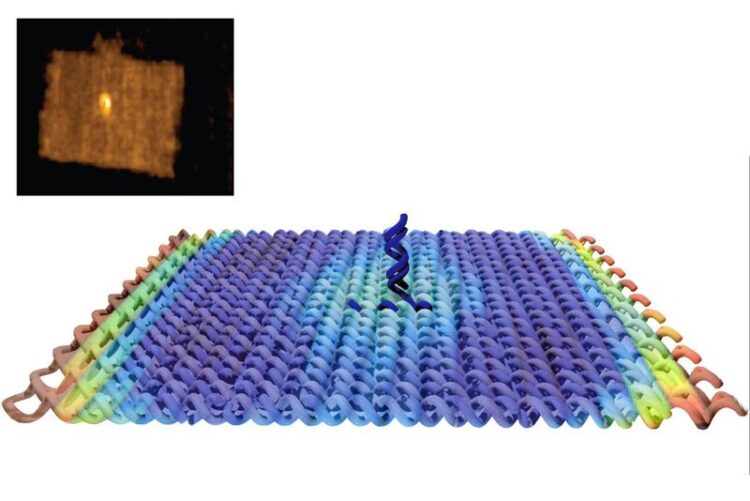
A molecular raft - created using the "origami"-technique.
Credit: TU Wien
A team at TU Wien was able to answer important questions about the immune system – with a trick reminiscent of paper folding.
T-cells are an important component of our immune system: with the receptors they carry on their surface, they can recognise highly specific antigens. Upon detection of an intruder, an immune response is triggered. It is still unclear exactly what happens when antigens are recognised: How many antigens are necessary to elicit an immune response, and does the response depend on their spatial arrangement?
These effects take place in the nanometer range – on the size scale of molecules, far below what can be seen with ordinary microscopes. To study all this, tiny tools are needed. Therefore, an unusual method was used at TU Wien: DNA molecules were folded in an ingenious way, similar to the paper folding art origami. In this way, not just a double helix is created, but a rectangular “molecular raft” that floats across a cell membrane and serves as a tool for novel measurements. The results have now been published in the scientific journal PNAS.
Artificial cell membranes
“T cells react to antigens presented by specific cells on their surface. To be able to study this interaction between the T-cells and the antigen-presenting cells in detail, we replace the antigen-presenting cell with an artificial cell membrane. This allows us to control the number and type of antigens ourselves,” says Prof. Eva Sevcsik, biophysicist at the Institute of Applied Physics at TU Wien.
“There was some evidence that the spatial distance between antigens plays an important role in T-cell activation,” says Joschka Hellmeier, who did research on this project as part of his dissertation. “However, it is difficult to study these effects precisely: The distance between the individual antigens is not so easy to determine.”
The cell membrane is not a fixed structure where every molecule stays in place. The antigens in the cell membrane can move freely, much like inflatable plastic toys floating on a water surface. “Therefore, we wanted to establish a method to precisely set certain distances between antigens and then study the reaction of the T-cells,” Eva Sevcsik explains.
DNA origami
To do this, the researchers made use of an important natural phenomenon: DNA, the carrier of genetic information in our body, consists of two precisely matching single strands that join together without external intervention to form a DNA double helix.
This property is exploited in DNA nanotechnology: “By cleverly designing single strands that only fit together in certain sections, you can connect several double helices with each other and thus create complicated structures,” explains Eva Sevcsik. “This technique is called DNA origami – instead of folding paper, we fold DNA strands.”
In this way, the research team built rectangular DNA platforms to which one can fix an antigen. This DNA rectangle is placed on the artificial membrane and it moves there like a raft.
“This way we can guarantee that the antigens do not come arbitrarily close to each other,” says Joschka Hellmeier. “Even if two of these DNA rafts move close together, there is still a minimum distance between the antigens if only one antigen is fixed on each DNA raft.” In addition, it is possible to built DNA raft variants each carrying two antigens at the same time. That way it is possible to study how the T-cells react to different antigen spacing.
Old riddle solved
Using this strategy, they were able to explain the contradictory observations that had caused confusion in the field of molecular immunology in recent years: sometimes, several neighbouring antigens seemed to be necessary to activate T-cells, in other cases, a single one was sufficient. “With the help of our DNA origami technique, we were able to clarify the role of molecular distances for T-cell activation,” says Eva Sevcsik.
For naturally occurring antigens, the distance does not matter – they act “solo” and are thus very efficient in T-cell activation. In research, however, instead of antigens, artificial T-cell activators are often used that bind particularly strongly to the T-cell receptor – and in this case at least two neighbouring molecules are needed to activate the T-cell. “This is an important result,” says Eva Sevcsik. “We were able to show for the first time that there are two different mechanisms here, this will play an important role for future studies and the development of T-cell-based immunotherapies used to treat cancer.”
###
Contact
Prof. Eva Sevcsik
Institute for Applied Physics
TU Wien
+43 1 58801 134898
eva.sevcsik@tuwien.ac.at
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



