Phosphorus Rubber
Goodyear’s 1839 discovery of the vulcanization of natural rubber obtained from rubber trees marks the beginning of the modern rubber industry. A variety of synthetic rubber products were subsequently developed. In the journal Angewandte Chemie,scientists have now introduced a new, interesting variant: a phosphorus-containing rubber with a structure that corresponds to that of natural rubber.
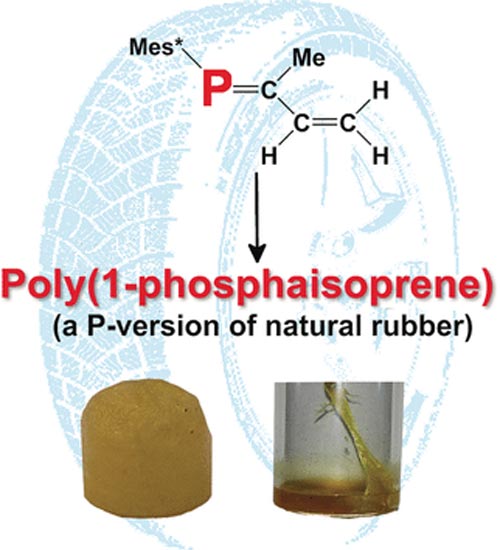
The similar properties of double bonds between carbon atoms (C=C) and phosphorus–carbon double bonds (P=C) led to the idea to try general polymerization techniques on the latter. After a number of successful attempts, researchers working with Derek P. Gates at the University of British Columbia (Vancouver, Canada) wanted to apply this concept to molecules that contain both P=C and C=C double bonds: phosphorus analogs of the building block of rubber, isoprene (2-methylbuta-1,3-diene) and its close relative, 1,3-butadiene.
Starting with phosphorus-containing precursors, the team was able to synthesize the first examples of poly(1-phospha-isoprene) and poly(1-phospha-1,3-butadiene). Precise characterization with a variety of spectrometric techniques gave some insight into the molecular structures of the resulting polymers. Like in the polymerization of isoprene and related dienes (compounds with two carbon-carbon double bonds), one of the double bonds in each building block is retained.
The polymerization mainly occurs through the C=C double bonds and only a tiny proportion happens at the P=C double bonds. This means that only a few phosphorus atoms are incorporated into the polymer backbone. The majority of the phosphorus atoms form side chains in which the P=C double bonds are maintained, leaving them available for further reactions or alterations to the polymers.
“Our functional phosphorus-containing materials are rare examples of polymers containing phosphaalkene moieties and offer many prospects for further derivatization and crosslinking,” according to Gates. For example, the researchers were able to bind gold ions to the polymers.
“As a macromolecular ligand for gold ions, the new polymers may be of future interest in catalysis and nanochemistry. Furthermore, the successful polymerization of P=C/C=C hybrid monomers opens the door to incorporate P-functionalities into commercial rubbers such as butyl rubber or styrene-butadiene rubber that traditionally use isoprene or butadiene comonomers. Such new copolymers promise unique architectures, properties, and functionality when compared to their carbon-only analogues.”
Dr. Derek P. Gates is Professor of Chemistry at the University of British Columbia. Over the past 18 years, Gates and his team have been working on the development of methods to create new phosphorus-containing polymers that are of interest for their novel flame retardant, catalytic, and sensor properties. He is the recipient of the CSC–Strem Chemicals Award for Pure or Applied Inorganic Chemistry.
Author: Derek P. Gates, University of British Columbia (Canada), https://www.chem.ubc.ca/derek-gates
Title: Polymerization of 1-Phosphaisoprene: Synthesis and Characterization of a Chemically Functional Phosphorus Version of Natural Rubber
Angewandte Chemie International Edition
Permalink to the original article: https://doi.org/10.1002/anie.201703590 – Please use in your news piece to make sure altmetric.com picks it up and a link to your piece is shown on the journal's website.
Copy free of charge. We would appreciate a transcript of your article or a reference to it.
The original article is available from our online pressroom at http://pressroom.angewandte.org.
Contact: Editorial office: angewandte@wiley-vch.de
To be removed from this list, please e-mail us.
Angewandte Chemie is a journal of the Gesellschaft Deutscher Chemiker (German Chemical Society, GDCh) and is published by Wiley-VCH. It is one of the prime chemistry journals in the world. Celebrate the society's 150th anniversary with us and eminent speakers, among them four Nobel Laureates.
Angewandte Chemie International Edition, Postfach 101161, 69451 Weinheim, Germany.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



