Backstage with a command performer
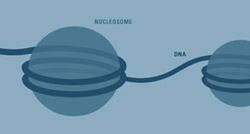
The chromatin of our cells’ nuclei consist of DNA "threads" wrapped around protein "spools." Each threaded spool is called a nucleosome. Rockefeller researchers led by Sasha Tarakhovsky discovered that one of the proteins, histone H3, in the spools of a B cell’s nucleus, is modified at an early stage of the cell’s maturation process. This necessary change to the chromatin accounts for much of the immune cell’s repertoire of possible antibodies, and is a first in understanding the functional effects of chromatin changes.
B cell chromatin study strikes physiological chord
Some cells sing with the chorus, while others unwittingly achieve fame on their own. The immune system’s B cell is a true diva that spends its early days preparing for the ultimate audition. Its repertoire of possible antibodies to invading microbes totals 50 million. For the immune system, this repertoire means the difference between destroying a potentially lethal antigen or not.
Since the late 1970s, the genes for making immunoglobulin, a family of blood proteins that compose the antibodies, sufficed to explain the B cell’s vast oeuvre. A B cell that is mature enough to respond to antigen does so by combining genes in a process called immunoglobulin gene rearrangement. Many possible combinations during this process allow a wide catalog of antibodies to literally take shape. Now, a biochemical phenomenon involving changes to stationary proteins in the B cell’s nucleus, called histones, is known also to contribute to the cell’s various solo performances.
In the February 2003 issue of Nature Immunology, Sasha Tarakhovsky and his Rockefeller University colleagues reveal that a little-studied regulating protein, Ezh2, carries out an important mission on histone H3 protein in developing B cells. In other words, a new criterion defining B cells’ uniqueness has been discovered.
The finding, while vitally important to understanding the B cell and its immune system counterparts, represents a first in determining the physiological effects of changes wrought on the chromatin fiber – the material basis of chromosomes made of DNA “thread” wound on protein “spools” called histones.
“We knew we could use our existing genetic tools to move from studying B cell signaling at the antigen receptor on the cell’s surface to that occurring in the cell’s nucleus,” says Tarakhovsky. “Ezh2 in early B cell development means the difference between 50 million antibody possibilities versus 50,000 in mice without Ezh2.”
A biochemical theory bears physiological fruit
Scientists were somewhat puzzled when the total number of genes in the human genome amounted to about 30,000, less than half the predicted amount. Turns out, DNA – that ticker tape of base pairings that chemically describes us – joins combinatorial forces with an assembly of proteins, called histones. The proteins form an octomer, or group of eight, that, together with two loops of DNA thread, form a structure called a nucleosome. These nucleosomes look like beads on a string, which is the chromatin. Effector proteins that change the chromatin structure depending on their precise biochemistry constantly are modifying histone proteins.
At Rockefeller, a few labs try to decipher DNA and histone changes, and the resulting “on-off” states of genes known as transcriptional regulation.
Perhaps best known for their work on gene regulation and transcriptional control, cutting the two sides of the chromatin cloth, are Rockefeller professors James Darnell and Robert Roeder, respectively.
Another is perhaps the world’s most well known histone scientist, David Allis. In 2001, Allis and colleague Thomas Jenuwein proposed the histone code theory in a paper published in Science magazine. Molecular biologists and geneticists have been abuzz with the proposal ever since. Allis, who joins The Rockefeller University as head of a new laboratory in March 2003, places great value on the work of Tarakhovsky and colleagues:
“There have been hints that programmed DNA rearrangements, including V(D)J recombination, are governed at some level by chromatin alterations, but these pathways are relatively unexplored and poorly understood,” says Allis. “It is now becoming clear that chromatin modifications influence gene expression, genomic stability, recombination and repair. Ultimately, all of this must influence cellular differentiation, development, and cell-type specificity.”
If life is a symphony of biochemical signaling, Allis and Jenuwein’s important proposal about histone proteins provides us with the sheet music. How the genome is played like a musical instrument – that is, which genes play their notes in an ongoing melody — depends on understanding the biochemical range of DNA and histone proteins, which chromatin researchers attempt to do. Allis and his colleagues know that understanding this range will tell us how it affects the functioning of living organisms. Tarakhovsky and his colleagues are applying the biochemical occurrences to the physiology of a cell and its impact on a living organism. They’re interpreting the music as it is played.
Allis adds: “It is an exciting field and it is wonderful to see the new laboratories turning to chromatin in their biological systems.”
Not an Ez mark
The notion that a nascent set of biochemical criteria helps us understand health and disease states is not farfetched. The challenge however, is to carve away at the chemical signals, delivered in the form of proteins and enzymes, responsible for those states.
Ezh2, a member of the polycomb family of proteins – the proteins generally known to silence the expression of genes during embryonic development — is not well defined. Tarakhovsky and I-hsin Su, a postdoctoral fellow and first author on the publication, only stumbled upon it.
“We became interested in this protein, Ezh2, because it binds to a potent signaling protein called Vav,” says Tarakhovsky. “Since we already knew that Vav plays a critical role in B and T cell function, and we wanted to know the molecular mechanism of Vav and its partners, Ezh2 became one of our investigative goals.”
Tracking Ezh2’s reception by the nucleosomes, which function like tiny nuclear satellite receivers, was not easy. When the researchers began their experiments, several years ago, the histone code, which involves several crucial chemical modification processes – acetylation, phosphorylation, methylation and ubiquitination – was not well understood. These modification processes form part of the combinatorial “code” of histone proteins’ interaction with DNA. Now, thanks to Su’s stamina on the Ezh2 inquiry, other labs likely will follow the same footsteps to study effector molecules.
“We thought that Ezh2 might serve as a direct link from receptor signaling to nuclear responses,” says Tarakhovsky. “But proof for Ezh2 as a methyltransferase, which we learned that it is, simply did not exist prior to our studies.” Su, Tarkahovsky and their colleagues studied how Ezh2 does its job in the B cell. What they found, by using a special kind of knockout mouse, and a novel mass spectroscopy analysis is that Ezh2 controls methylation of histone H3 on one particular lysine residue, or biochemical modification site, on what is known as the histone “tail.”
Conditional, not conventional
Immunologists were among the vanguard of molecular biologists to tinker with the genes of the mouse. Early on, naturally occurring mutant mouse strains indicated the ability to probe the immune system by artificially deleting or adding a gene. Unfortunately, many genes that could be “knocked in” or “knocked out” are critical for the embryonic or early postnatal development. Creating a conventional knock out animal, in which a gene is eliminated from the beginning, would not allow the analysis of the immune system because embryological development could not progress without the gene.
Between 1989 and 1994, when genetically altered mice gained popularity as a powerful new research tool, immunologists developed another kind of genetic alteration system in mice in order to solve the problem of how to study a gene by knocking it out when it also plays a role in development. In 1994, a Cologne-based group headed by Klaus Rajewsky generated the first tissue-specific knockout mouse.
Tarakhovsky, a professor at the University of Cologne before coming to Rockefeller, has successfully applied this tissue-specific gene modification technique, call “Cre-loxP conditional mutagenesis,” to create animals that lack specific enzymes or other proteins in lymphocytes. Tarakhovsky’s lab uses these specially tailored mice in almost all of their projects.
“Like other target molecules, Ezh2, is required during development. A conventional knockout would blunt the point of our investigation,” says Tarakhovsky.
The researchers instead embarked on creating Cre-loxP conditional knock out mice for Ezh2. The technique involves two transgenic mouse strains, one carries Cre, an enzyme, under the control of a B or T cell promoter gene. The other strain, in this case, carries the Ezh2 gene surrounded by a special binding site called “loxP”. LoxP sites on DNA are recognizable by the Cre enzyme. When Cre mice are bred with mice carrying the loxP surrounded Ezh2 gene, the resulting mice lack Ezh2 gene only in developing B cells. As a result the Ezh2 needed in embryonic development is not deterred in these mice. This in vivo model of studying cell signaling is invaluable.
B cells have a distinct melody, as all cells do. Cellular proteins and enzymes striking the chords of the genome and histones inside the cell’s nucleus play the tune. Without the groundbreaking work of Allis and his colleagues, Tarakhovsky’s results may have remained unclear. Without the masterful ability to create conditional changes in a living organism, what we know about the histone code could not be easily linked to the symphony of life.
Founded by John D. Rockefeller in 1901, The Rockefeller University was this nation’s first biomedical research university. Today it is internationally renowned for research and graduate education in the biomedical sciences, chemistry, bioinformatics and physics. A total of 21 scientists associated with the university have received the Nobel Prize in medicine and physiology or chemistry, 16 Rockefeller scientists have received Lasker Awards, have been named MacArthur Fellows and 11 have garnered the National Medical of Science. More than a third of the current facultyare elected members of the National Academy of Sciences.
Media Contact
More Information:
http://www.rockefeller.edu/pubinfo/021803.phpAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



