Cancer vaccine based on pathogenic listeria bacteria shows promise targeting metastases
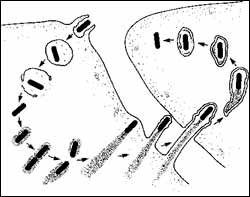
Upon entering a cell, the listeria bacterium takes over the cellular machinery and builds a rocket sled that propels it out of the cell and into another, spreading the infection.
An experimental cancer vaccine using defanged listeria bacteria is showing great promise in animal studies, successfully treating new cancers that have spread into the lungs of mice.
The mouse study, reported in the Sept. 21 issue of the journal Proceedings of the National Academy of Sciences by scientists at Cerus Corp. in Concord, Calif., employs a genetically engineered listeria bacteria based on a strain created by coauthor and University of California, Berkeley, microbiologist Daniel Portnoy. Buoyed by the success of the new cancer vaccine, Cerus scientists now are aiming for human trials.
Cerus ultimately hopes to use the genetically engineered listeria vaccine to target cancers such as pancreatic and ovarian cancer, and possibly leukemia and various solid tumors. The listeria bacteria are uniquely effective vehicles for a cancer vaccine, Portnoy said, because the bacteria incite a strong “innate” response from the immune system and at the same time sneak cancer antigens into cells to optimally stimulate a potent “acquired” immune response. Together, these two independent arms of the human immune system can deliver a one-two punch to cancer cells. Antigens are like a red flag to a bull – they draw an attack from cells of the immune system – but the strength of the immune response depends on how the flag is waved.
“This immune therapy uses bacteria that induce both inflammation and an immune response to specific tumor antigens,” which together hit the tumor with generalized antitumor chemicals, such as interferon and tumor necrosis factor, as well as activated T-cells that attack and kill the tumor, Portnoy said. “Listeria is the best bug so far to induce that response. With listeria, innate and acquired immunity work in concert.”
Portnoy, a professor of molecular and cell biology and of public health and a member of UC Berkeley’s Health Sciences Initiative, identified the genes in listeria that make it virulent and, with Cerus scientists, knocked them out. The modified bacteria ignite a full-blown “innate” immune response in the vaccinated mice, but with a thousand-fold less toxicity than the wild bacteria.
Cerus then took the genetically engineered listeria and inserted cancer antigens, again using a technique developed by Portnoy, then infused the vaccine into the mice. All showed tumor regression and reduced lung metastases, and 40 percent of the mice were long-term survivors. “These studies address one of the major barriers to using listeria therapeutically, which is, how you create a strain that is safe enough to move forward into the clinic,” said David Cook, vice president of research and development for Cerus. “With this strain, we are now moving from essentially pure research to developing clinical applications, planning for manufacturing and performing toxicity studies to submit to the FDA.”
One hope for this and other cancer vaccines is that they will stimulate the immune system to seek out metastatic cancer cells that have been shed by the original tumor and kill them before they can establish themselves elsewhere in the body. “The first place to start is to treat patients with minimal residual disease, that is, use the vaccine to prevent recurrence after surgery and chemotherapy,” Cook said. Ovarian cancer is a good example of a tumor that has a high recurrence rate that could potentially be knocked back with a listeria-based vaccine.
Listeria is ideal as a vehicle for cancer vaccines because it normally induces a strong innate immune response in humans as well as a potent induced immune response. The innate response is a generalized attack on any invader, characterized by secretion of chemicals called cytokines – including tumor necrosis factor and interferon – that mobilize generalized killing machines such as macrophages, neutrophils and natural killer cells.
Mammals have, in addition, an adaptive response that targets specific invaders. This involves antibody-producing B cells and cancer-killing T cells mobilized against antigens, which are molecular targets expressed by microbes, viruses or cancer cells. One of the problems with cancer antigens is that that cancer cells look like the body’s own cells, and so the immune system doesn’t attack them. Listeria breaks that “self tolerance” and mobilizes the T cells to recognize and attack cancer cells expressing these antigens.
It is not yet completely clear what makes listeria such a good inducer of immunity, Portnoy said, but it probably has to do with its cell biology, the fact that it grows directly in the cell cytosol. There it stimulates the one pathway that produces cytotoxic T cells – often called CD8+ cells – that can attack and destroy tumors. Portnoy recently showed that live, but not killed, listeria induce the production of beta-interferon, which may explain this effect. “The unique ability of listeria to get into the cytosol is the key to its effectiveness,” Cook added. “Listeria not only stimulates this additional innate immune response, but it also puts the antigen into exactly the pathway we want and gives us a robust antigen-specific response.”
Listeria also can avoid antibodies, whereas other microbial vectors set up an antibody response that prevents them from being used more than once. A listeria-based cancer vaccine could thus be given periodically until a tumor has responded.
Despite these attributes, the one problem with listeria has been toxicity. Though a relatively rare disease, listeriosis has a high fatality rate of between 20 and 30 percent. It made headlines two years ago when 20 people, including several children, died of listeriosis from tainted hamburger, forcing the largest meat recall in history. Several thousand people come down with listeriosis each year, and several hundred die from it.
But the disease kills primarily those with compromised immune systems; in most people, it is quickly knocked down by the immune system. The challenge with turning listeria into a vaccine was to retain its ability to stimulate a strong immune system attack, yet reduce its ability to spread from cell to cell and cause toxicity, Portnoy said.
Listeria bacteria establish an infection by inducing immune system cells, mostly scavenger cells called phagocytes, to engulf them, so that they end up encased in a bubble within the body of the cell. The bacteria would be benign if they remained isolated in this compartment, because the cell can kill them there. But they are able to break out into the cell interior, the cytosol, and take over the host cell’s machinery to spread the infection.
Once hooked into the cell’s machinery, the bacteria spread in an amazing fashion. They generate comet-like tails that push them around the cell like a speedboat. Eventually, they slam into the cell membrane and pop from one cell into the next, spreading the infection.
Six years ago, Matthew D. Welch, an associate professor of molecular and cell biology at UC Berkeley, and his colleagues discovered that the tail is produced when ActA, a protein on the surface of the bacteria, interacts with a complex of host cell proteins called the Arp2/3 complex. The result is rapid polymerization of a skeletal protein called actin that piles up and physically propels the newly formed bacteria around the cell.
Portnoy thought that if he could eliminate the gene for ActA, he could prevent the bacteria from forming actin motors and spreading from cell to cell. The technique worked, and Cerus adapted one of Portnoy’s attenuated strains for its vaccine. The particular strain Cerus plans to use for its clinical trials is missing not only ActA but another gene, InlB, which encodes a protein called Internalin B. This mutation was made at Cerus using Portnoy’s methods in order to prevent direct invasion of liver cells, thus dramatically lowering liver damage by the vaccine strain.
Cerus plans to use this doubly attenuated listeria strain as a platform for various types of cancer vaccines. In fact, Cook said, as long as an antigen can be found that stimulates an immune system attack against a cancer, that antigen could be inserted into listeria to create a vaccine.
At the moment, Cerus’ preclinical development programs are focused on two novel cancer antigens, according to a recent company press release. Cerus is collaborating with Johns Hopkins University to develop a listeria-based vaccine targeting pancreatic and ovarian cancers that express the Mesothelin antigen, and has partnered with MedImmune, Inc., to develop a listeria-based vaccine targeting the large number of tumors, including breast, prostate and melanoma, that overexpress the antigen EphA2. Both antigen targets have recently been featured in new research reports suggesting their significance as potential targets for cancer treatment.
The listeria-based cancer vaccine work was sponsored by Cerus Corp., with support for Portnoy from the U.S. Public Health Service. Portnoy also serves as a consultant for Cerus. Coauthors on the paper are Dirk Brockstedt, Martin Giedlin, Meredith Leong, Keith Bahjat, Yi Gao, William Luckett, Weiqun Liu and Thoms Dubensky Jr. of Cerus.
Media Contact
More Information:
http://www.berkeley.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



