Scientists achieve self-assembly of genertically engineered spider silk fibre in insect cells
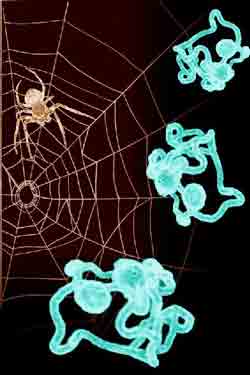
Illustration shows spider spinning its web (left) and laboratory-produced "spider" fibers grown in insect cells (right).
For the first time anywhere, scientists from the Hebrew University of Jerusalem and from Germany have succeeded in producing self-assembled spider web fibres under laboratory conditions, outside of the bodies of spiders. This fibre is significantly stronger than the silk fibre made by silkworms.
The achievement by the research team, described in an article in the Nov. 23 issue of Current Biology, opens the way to commercial development of this spider fibre for numerous industrial applications.
Silk has been in use by mankind for thousands of years. However, unlike silkworms, spiders are territorial in nature and thus not subject to domestication and commercial growth in quantities.
Scientists have attempted to create spider’s webs independently of the spider itself through genetic engineering by manufacturing the proteins, which constitute the silk fibres of the webs, through the use of bacteria, yeast, plants and mammalian cells in tissue culture. But these efforts were unsuccessful in producing fibres with properties similar to the natural ones.
Now, the Israeli-German scientific team has succeeded, through techniques of genetic engineering, in creating spontaneous production of spider web fibre in insect cell cultures. These fibres were equal in their chemical resistance characteristics to those produced by the spider. Mass production of such fibre in the future can be used industrially in various areas which require fine applications. The Yissum Research Development Company of the Hebrew University and German partners are focusing on commercializing the research.
Participants in the research, which has been conducted over the past two years, are the developmental biologist Dr Uri Gat, doctoral student Shmulik Ittah and research assistant Shulamit Cohen of the Department of Cell and Animal Biology in the Silberman Institute of Life Sciences at the Hebrew University; Dr Thomas Scheibel and doctoral student Daniel Huemmerich, biophysicists at the Technical University of Munich; and Fritz Vollrath of Oxford University.
Spider webs consist of fibres (spider silks) produced by specific proteins. In order to artificially synthesize these proteins, the researchers utilised sections of the genes of the garden spider (Araneus diadematus), which are involved in the manufacture of these proteins.
The spider spins its web from various types of fibres, including the fibre known as dragline silk, which is characterized by great strength and elasticity. It is six times stronger than nylon and steel fibre of equal diameter and serves the spider as a “lifeline” in case of falling. This fibre is made up primarily from two proteins, ADF-3 and ADF-4, which are genetically similar and are produced in a gland in the abdomen of the spider. The process by which these proteins pass from the moment of their production until their excretion as fibre was not understood until now.
In their laboratory experiments, the researchers introduced the genes, which encode the two dragline silk proteins, into an insect-infecting virus, known as baculovirus. These genetically engineered viruses were then grown in cultures of cells derived from a type of caterpillar called the fall armyworm.
“Since spiders and insects are both arthropods and since their genomes are more closely related to each other than to those creatures with which prior experiments were conducted, we felt that we would be able to produce spider fibres using these insects,” said Dr Gat. “For this purpose, we developed a methodology for producing great quantities of the appropriate proteins, which is based on infecting the insect cells with the genetically engineered virus, in order to produce the fibre.”
After the engineered viruses infected the insect cells, the cells began producing the proteins, and subsequently “spider” fibres spontaneously formed in them. However, – unlike in spiders – these laboratory-produced fibres were made up only of the ADF-4 protein, while the ADF-3 protein remained dissolved. Nevertheless, these fibres were identical in their diameter to that of real spider fibre and were found to be equal to — and in certain aspects even exceed — the chemical resistance quality of the spider-created fibre.
The scientists believe that that the variability in the behaviour of the proteins they produced as compared to what occurs in nature shows a high level of sophistication in the spider fibres. It seems that the protein ADF-4 makes it possible for the rapid production of fibre, while the other protein, ADF-3, regulates production and prevents early fibre production, which could be fatal to the spider.
The researchers are now hoping to be able to create conditions, which will make it possible to produce the spider fibres in quantity without the limitations of having to do this within insect cells.
“The research enabled us to determine the close connection that exists between the sequence, structure and functions of the proteins,” said Dr Gat. “From a practical viewpoint, mass production of fibres, whose diameter is one-thousandth of a millimeter, is likely to be useful in the future for manufacture of bulletproof vests, surgical thread, micro-conductors, optical fibres and fishing rods; even new types of clothing may be envisioned.”
Media Contact
More Information:
http://www.huji.ac.ilAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

NTU and NUS spin-off cutting-edge quantum control technology
AQSolotl’s quantum controller is designed to be adaptable, scalable and cost-efficient. Quantum technology jointly developed at Nanyang Technological University, Singapore (NTU Singapore) and National University of Singapore (NUS) has now…

How Geothermal Energy Shapes Bavaria’s Green Future Through Sustainable Energy
The Bavarian State Ministry of Science and the Arts has extended its funding for the research association “Geothermal Alliance Bavaria,” with the University of Bayreuth (UBT) continuing as a member…

Spintronics memory innovation: A new perpendicular magnetized film
Long gone are the days where all our data could fit on a two-megabyte floppy disk. In today’s information-based society, the increasing volume of information being handled demands that we…



