Picky female frogs drive evolution of new species in less than 8,000 years
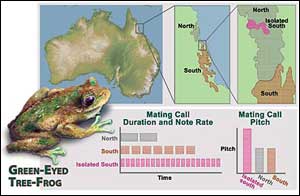
When isolated populations of the green-eyed tree frog (gray and brown) met again 8,000 years ago, they found that each had changed in subtle ways. The calls of the male frogs were different, and more importantly, hybrid offspring were less viable. One population that was cut off from its southern kin (pink) found a way to ensure healthy young. Females, who choose mates based only on their call, began selecting mates with a the southern call type. Over thousands of years, this behavior exaggerated the pre-existing differences in call, lead to smaller body size in males of the "isolated southern population" and resulted in rapid speciation between the two populations of the southern lineage (pink and brown). (Nicolle Rager Fuller/National Science Foundation)
Picky female frogs in a tiny rainforest outpost of Australia have driven the evolution of a new species in 8,000 years or less, according to scientists from the University of Queensland, the University of California, Berkeley, and the Queensland Parks and Wildlife Service.
“That’s lightning-fast,” said co-author Craig Moritz, professor of integrative biology at UC Berkeley and director of the Museum of Vertebrate Zoology. “To find a recently evolved species like this is exceptional, at least in my experience.”
The yet-to-be- named species arose after two isolated populations of the green-eyed tree frog reestablished contact less than 8,000 years ago and found that their hybrid offspring were less viable. To avoid hybridizing with the wrong frogs and ensure healthy offspring, one group of females preferentially chose mates from their own lineage. Over several thousand years, this behavior created a reproductively isolated population – essentially a new species – that is unable to mate with either of the original frog populations.
This example suggests that rapid speciation is often driven by recontact between long-isolated populations, Moritz said. Random drift between isolated populations can produce small variations over millions of years, whereas recontact can amplify the difference over several thousands of years to generate a distinct species.
“The overarching question is: Why are there so many species in the tropics?” Moritz said. “This work has led me to think that the reason is complex topography with lots of valleys and steep slopes, where you have species meeting in lots of little pockets, so that you get all these independent evolutionary experiments going on. Perhaps that helps explain why places like the Andes are so extraordinarily diverse.”
Moritz; lead author Conrad Hoskin, a graduate student at the University of Queensland in St. Lucia, Australia; and colleagues Megan Higgie of the University of Queensland and Keith McDonald of the Queensland Parks and Wildlife Service, reported their findings in the Oct. 27 issue of Nature.
The green-eyed tree frog, Litoria genimaculata, lives in the Wet Tropics area of northeast Queensland, a rugged tropical region of Australia along the Pacific Ocean’s Great Barrier Reef. The frog, which is green with reddish-brown splotches, is common around streams and grows to about 2 1/2 inches in length.
Because of geographic isolation that began between 1 and 2 million years ago with the retreat of rainforest to higher elevations, two separate frog lineages developed in the northern and southern parts of the species’ coastal range – only to be reconnected less than 8,000 years ago as the climate got wetter and warmer and the rainforest expanded.
Hoskin and his colleagues found that the northern and southern calls of the male frog, which are what females pay attention to in the mating game, had become different from each other. Yet despite this difference, reflected in the call’s duration, note rate and dominant frequency, the two lineages could still breed with one another.
The southern females, however, were more picky about their mates than the northern females. And in one area of contact that had become isolated from the southern range, the southern females were extremely picky, to the extent that they almost never mated with northern males.
In laboratory breeding experiments, the biologists discovered the reason for this choosiness: While northern and southern lineages could breed successfully, they apparently had diverged enough during their million-year separation that offspring of southern females and northern males failed to develop beyond the tadpole stage. Though crosses involving northern females and southern males successfully produced frogs, the offspring developed more slowly than the offspring of pairs of northern frogs.
Field studies confirmed the laboratory results. Researchers could find no hybrid frogs in the contact zones that were the offspring of southern mothers, judging by the absence of any southern mitochondrial DNA, which is contributed only by the mother.
Hoskin and colleagues argue that because southern females have the most to lose in such cross-breeding, there may have been selection pressure to evolve a mating strategy to minimize dead-end mating with northern males. This appears to have occurred in the contact region where a population of the southern lineage had become isolated from the rest of its lineage and had developed a preference for certain male calls. The male frog call in this population has diverged significantly from both the northern and southern lineage calls.
“If females have a reason not to get the mating wrong, and they have some way of telling the males apart – the call – the theory is that this should create evolutionary pressure for the female choice to evolve so that they pick the right males,” Moritz said.
This so-called reinforcement has been controversial since the time of Charles Darwin, with some biologists claiming that it requires too many steps for evolution to get it right.
“Some have argued that it’s just too complicated and that it is not really necessary, and there have been few convincing demonstrations. In their view, differences between populations arise because of natural selection or genetic drift or mutation or some combination of those three, and reproductive isolation is just some glorious accident that arises from that,” Moritz said. “We do have very compelling evidence. We have addressed various lines of evidence and conclude that there has been reinforcement and that has given rise to a new species based on very strong female choice.”
As a comparison, they looked at a second contact zone on the border between north and south, where frogs were not isolated from either lineage.
“Reinforcement does not appear to occur at the more ’classic’ contact between northern and southern lineages, and we speculate that this may be due to gene flow from the extensive range of the southern lineage into the contact zone,” Hoskin said. “This problem does not exist at the other contact because the southern lineage population is very small and occurs primarily within the contact zone.”
Because the frogs in the isolated contact area had a distinctively different call, and because they were effectively isolated from surrounding populations by mating preference, Hoskin and colleagues concluded that female choice led to this new species.
Interestingly, evolutionary theory would predict that the southern and northern frog populations would drift apart into two distinct species. In the case of the green-eyed tree frog, Moritz said, a subpopulation of the southern species drifted away not only from the northern species, but also from the southern. That was unexpected, he said.
Moritz noted that geographic isolation in this “dinky bit of rainforest in Australia” has split many species, and that reinforcement at zones of recontact may be generating other new species.
“In this tropical system, we have had long periods of isolation between populations, and each one, when they come back together, have got a separate evolutionary experiment going on. And some of those pan out and some don’t. But if they head off in different directions, the products themselves can be new species. And I think that’s kinda cool. It gives us a mechanism for very rapid speciation.”
The research was supported by the U.S. National Science Foundation, the University of Queensland and the Australian Cooperative Research Centre for Tropical Rainforest Ecology and Management.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

How marine worms regenerate lost body parts
The return of cells to a stem cell-like state as the key to regeneration. Many living organisms are able to regenerate damaged or lost tissue, but why some are particularly…

Nano-scale molecular detective
New on-chip device uses exotic light rays in 2D material to detect molecules. Researchers have developed a highly sensitive detector for identifying molecules via their infrared vibrational “fingerprint”. Published in Nature…

Novel CAR T-cell therapy
… demonstrates efficacy and safety in preclinical models of HER2-positive solid tumors. The p95HER2 protein is found expressed in one third of HER2+ tumors, which represent 4% of all tumors….



