Right program could turn immune cells into cancer killers
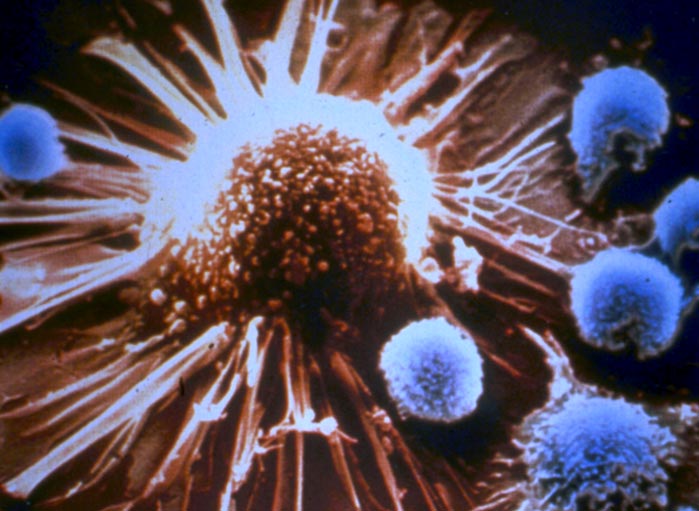
A tumor-specific T cell engages with a tumor cell. Bystander T cells do not engage with the tumor.
Credit: PNAS Dec. 10, 2002, Copyright (2002) National Academy of Sciences, U.S.A.
Cancer-fighting T cells from patients whose cancers responded to immunotherapy and from those whose tumors did not respond showed marked differences in gene activity that could eventually serve as targets to boost effectiveness.
Cancer-fighting immune cells in patients with lung cancer whose tumors do not respond to immunotherapies appear to be running on a different “program” that makes them less effective than immune cells in patients whose cancers respond to these immune treatments, suggests a new study led by researchers at the Johns Hopkins Kimmel Cancer Center Bloomberg~Kimmel Institute for Cancer Immunotherapy.
The findings, published in the August 5 issue of Nature, could lead to new ways to overcome tumor resistance to these treatments.
“Cancer immunotherapies have tremendous promise, but this promise only comes to fruition for a fraction of patients who receive them,” says study leader Kellie N. Smith, Ph.D., assistant professor of oncology and Johns Hopkins Bloomberg~Kimmel Institute of Immunotherapy investigator. “Understanding why patients do or don’t respond could help us raise these numbers.”
Cancer immunotherapies have gained traction in recent years as a way to harness the immune system’s inherent drive to rid the body of malignant cells, Smith explains. One prominent type of immunotherapy, known as checkpoint inhibitors, breaks down molecular defenses that allow cancer cells to masquerade as healthy cells, enabling immune cells known as CD8 T cells to attack the cancer cells. Different populations of these immune cells recognize specific aberrant proteins, which prompt them to kill malignant cells as well as cells infected by various viruses.
Although checkpoint inhibitors have shown tremendous success in some cancer types — even sometimes eradicating all evidence of disease — the portion of patients with these dramatic responses is relatively low. For example, only about a quarter of patients with non-small cell lung cancer (NSCLC) have significant responses to these treatments.
Searching for differences between responders and nonresponders, Smith and her colleagues turned to results of a previous immunotherapy study. They gathered blood, tumor and healthy tissue samples taken from 20 early-stage NSCLC patients who took part in the previous study, which tested the effects of administering immune checkpoint inhibitors before surgery to remove tumors. Nine of the patients had a dramatic response to checkpoint inhibitors, with 10% or less of their original tumors remaining at the time of surgery. The other 11 patients were nonresponders and had either significantly lower responses or no response at all.
After isolating CD8 T cells from each of these samples, the researchers used a technology developed at Johns Hopkins called MANAFEST (Mutation Associated NeoAntigen Functional Expansion of Specific T cells) to search specifically for those cells that recognize proteins produced by cancerous mutations (known as mutation-associated neoantigens, or MANA), influenza or Epstein-Barr, the virus that causes infectious mononucleosis. They then analyzed these cells using a commercially available technique called single cell transcriptomics to see which genes were actively producing proteins in individual cells — the “program” that these cells run on.
The researchers found that responders and nonresponders alike had similarly sized armies of CD8 T cells in their tumors, with similar numbers of cells in both populations that respond to MANA, influenza and Epstein-Barr. However, when they compared the transcriptional programs between responders and nonresponders, they found marked differences. MANA-oriented CD8 T cells from responders showed fewer markers of exhaustion than those in nonresponders, Smith explains. Responders’ CD8 cells were ready to fight when exposed to tumor proteins and produced fewer proteins that inhibit their activity, she says. In one patient who showed a complete response to checkpoint inhibitors — no evidence of active cancer by the time of surgery — the MANA-oriented CD8 T cells had been completely reprogrammed to serve as effective cancer killers. In contrast, nonresponders’ MANA-oriented CD8 T cells were sluggish, with significantly more inhibitory proteins produced.
Both responders and nonresponders’ MANA-, influenza- or Epstein-Barr-oriented CD8 T cells had significant differences in their programming as well. The MANA-oriented cells tended to be incompletely activated compared with the other CD8 T cell types. The MANA-oriented cells were also significantly less responsive to interleukin-7, a molecule that readies immune cells to fight, compared with influenza-oriented cells.
Together, Smith says, these findings suggest numerous differences in MANA-oriented cells between checkpoint inhibitor responders and nonresponders that could eventually serve as drug targets to make nonresponders’ CD8 T cells act more like responders’ — both for NSCLC and a broad array of other cancer types.
“By learning how to reprogram these immune cells, we could someday facilitate disease-free survival for more people with cancer,” says Smith. She adds that “an important and interesting finding was that nonresponders had cells that recognized the tumor. So there is ‘hope’ for developing treatments for patients who don’t respond to single agent immunotherapy. We just need to figure out the right target to activate these cells to help them do what they were made to do.”
###
Other Johns Hopkins researchers who contributed to this study include Justina X. Caushi, Jiajia Zhang, Zhicheng Ji, Ajay Vaghasia, Boyang Zhang, Emily Han-Chung Hsiue, Brian J. Mog, Wenpin Hou, Richard Blosser, Ada Tam, Valsamo Anagnostou, Tricia R. Cottrell, Haidan Guo, Hok Yee Chan, Dipika Singh, Sampriti Thapa, Arbor G. Dykema, Poromendro Burman, Begum Choudhury, Luis Aparicio, Laurene S. Cheung, Mara Lanis, Zineb Belcaid, Margueritta El Asmar, Peter B. Illei, Rulin Wang, Jennifer Meyers, Kornel Schuebel, Anuj Gupta, Alyza Skaist, Sarah Wheelan, Jarushka Naidoo, Kristen A. Marrone, Malcolm Brock, Jinny Ha, Errol L. Bush, Matthew Bott, David R. Jones, Joshua E. Reuss, Victor E. Velculescu, Kenneth W. Kinzler, Shibin Zhou, Bert Vogelstein, Janis M. Taube, Julie R. Brahmer, Patrick M. Forde, Srinivasan Yegnasubramanian, Hongkai Ji, Drew M. Pardoll.
This work was funded by grants from the Lung Cancer Foundation of America, the Mark Foundation for Cancer Research, the SU2C/Mark Foundation Lung Cancer Dream Team Convergence Award, the SU2C INTIME and SU2C-LUNGevity-American Lung Association Lung Cancer Interception Dream Team, Bristol-Myers Squibb, the SU2C DCS International Translational Cancer Research Dream Team, The IASLC Foundation, Swim Across America, The LUNGevity Foundation, the Commonwealth Foundation, the Banks Family Foundation, Subsidies for Current Expenditures to Private Institutions of Higher Education from the PMAC, through a subaward from Juntendo University, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Virginia and D.K. Ludwig Fund for Cancer Research, the Ludwig Center for Cancer Immunotherapy at Memorial Sloan Kettering, Cancer Research Institute, the Parker Institute for Cancer Immunotherapy, the Lustgarten Foundation for Pancreatic Cancer Research, the Conquer Cancer Foundation of ASCO, Bloomberg Philanthropies, the Maryland Cigarette Restitution Fund, the V Foundation, the Allegheny Health Network–Johns Hopkins Research Fund, the Damon Runyon Cancer Research Foundation, U.S. National Institutes of Health grants (R37CA251447, R01HG010889, R01HG009518, R01CA121113, R01CA217169, R01CA240472, CA62924, T32 CA193145, T32 CA009110, and T32 GM136577), and National Institutes of Health Cancer Center Support Grants (P30 CA008748 and P30 CA006973).
Anagnostou receives research funding from Bristol-Myers Squibb and AstraZeneca. Taube receives research funding from Bristol-Myers Squibb and serves a consulting/advisory role for Bristol-Myers Squibb, Merck, and AstraZeneca. Illei receives research funding from Bristol-Myers Squibb and Erbe Elektromedizin GmbH and serves a consulting/advisory role for AstraZeneca and Veran Medical Technologies. Naidoo receives research funding from AstraZeneca, Bristol-Myers Squibb, and Merck, and serves a consulting/advisory role for AstraZeneca, Daiichi Sankyo, Bristol-Myers Squibb, Merck, and Roche/Genentech. Marrone is a consultant for Amgen and AstraZeneca. Jones is a consultant for More Health and AstraZeneca and a steering committee member for Merck. Park is a consultant for AstraZeneca and Regeneron and has received honoraria from Intuitive Surgical. Chaft is a consultant for AstraZeneca, Genentech, Merck, Flame Bioscience and Novartis. Velculescu is a founder of Delfi Diagnostics and Personal Genome Diagnostics, serves on the board of directors and as a consultant for both organizations, and owns Delfi Diagnostics and Personal Genome Diagnostics stock, which are subject to certain restrictions under university policy.
Additionally, The Johns Hopkins University owns equity in Delfi Diagnostics and Personal Genome Diagnostics. Velculescu is also an adviser to Bristol-Myers Squibb, Genentech, Merck, Takeda Pharmaceuticals, Daiichi Sankyo, Janssen Diagnostics and Ignyta. Hellman receives research support from Bristol-Myers Squibb; has been a compensated consultant for Merck, Bristol-Myers Squibb, AstraZeneca, Genentech/Roche, Nektar, Syndax, Mirati, Shattuck Labs, Immunai, Blueprint Medicines, Achilles, Arcus, and Natera; and has received travel support/honoraria from AstraZeneca, Eli Lilly and Bristol-Myers Squibb. He also has options from Shattuck Labs, Immunai and Arcus and has a patent filed by his institution related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx. The Johns Hopkins University is in the process of filing patent applications related to technologies described in this paper on which Hsiue, Vogelstein, Kinzler and Zhou are listed as inventors. Vogelstein and Kinzler are founders of Thrive Earlier Detection. Kinzler is a consultant to and was on the board of directors of Thrive Earlier Detection. Vogelstein, Kinzler and Zhou own equity in Exact Sciences; are founders of, hold or may hold equity in, and serve or may serve as consultants to manaT Bio, manaT Holdings, Personal Genome Diagnostics and NeoPhore. Zhou has a research agreement with BioMed Valley Discoveries. Kinzler and Vogelstein are consultants to Sysmex, Eisai and CAGE Pharma and hold equity in CAGE Pharma. Vogelstein is a consultant to and holds equity in Catalio. The companies named above, as well as other companies, have licensed previously described technologies related to the work from this lab at The Johns Hopkins University. Licenses to these technologies are or will be associated with equity or royalty payments to the inventors as well as to The Johns Hopkins University. Merghoub is a co-founder and holds equity in IMVAQ Therapeutics; is a consultant for Immunos Therapeutics, ImmunoGenesis and Pfizer; receives research funding from Bristol-Myers Squibb, Surface Oncology, Kyn Therapeutics, Infinity Pharmaceuticals, Inc., Peregrine Pharmaceuticals, Inc., Adaptive Biotechnologies, Leap Therapeutics, Inc. and Aprea; and holds patents on applications related to work on oncolytic viral therapy, alpha virus-based vaccines, neoantigen modeling, CD40, GITR, OX40, PD-1 and CTLA-4. Brahmer serves an advisory/consulting role for Amgen, AstraZeneca, Bristol-Myers Squibb, Genentech/Roche, Eli Lilly, GlaxoSmithKline, Merck, Sanofi and Regeneron; receives research funding from AstraZeneca, Bristol-Myers Squibb, Genentech/Roche, Merck, RAPT Therapeutics, Inc. and Revolution Medicines; and is on the data and safety monitoring board of GlaxoSmithKline, Janssen and Sanofi. P.M.F. receives research support from AstraZeneca, Bristol-Myers Squibb, Novartis, and Kyowa; has been a consultant for AstraZeneca, Amgen, Bristol-Myers Squibb, Daichii Sankyo, and Janssen; and serves on a data safety and monitoring board for Polaris. Yegnasubramanian receives research funding from Bristol-Myers Squibb/Celgene, Janssen and Cepheid, has served as a consultant for Cepheid, and owns founder’s equity in Astra Therapeutics and Digital Harmonic. Smith, Pardoll, Vogelstein and Kinzler have filed for patent protection on the MANAFEST technology described herein (serial no. 16/341,862). K.N.S., D.M.P., J.E.C., B.V., E.H.-C.H. D.M.P., K.W.K. and S.Z. have filed for patent protection on the p53 R248L mutation-specific TCR described herein (serial No. 63/168,878). Pardoll is a consultant for Compugen, Shattuck Labs, WindMIL, Tempest, Immunai, Bristol-Myers Squibb, Amgen, Janssen, Astellas, Rockspring Capital, Immunomic and Dracen, and owns founder’s equity in manaT Holdings, LLC, WindMIL, Trex, Jounce, Anara, Tizona, Tieza and RAPT, and receives research funding from Compugen, Bristol-Myers Squibb and Anara. Smith has received travel support/honoraria from Illumina, Inc., receives research funding from Bristol-Myers Squibb, Anara and Astra Zeneca, and owns founder’s equity in manaT Holdings, LLC. The terms of all these arrangements are being managed by the respective institutions in accordance with their conflict of interest policies.
Media Contact
Amy Mone
Johns Hopkins Medicine
amone@jhmi.edu
Office: 410-614-2915
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



