Sea urchin protein provides insights into self-assembly of skeletal structures
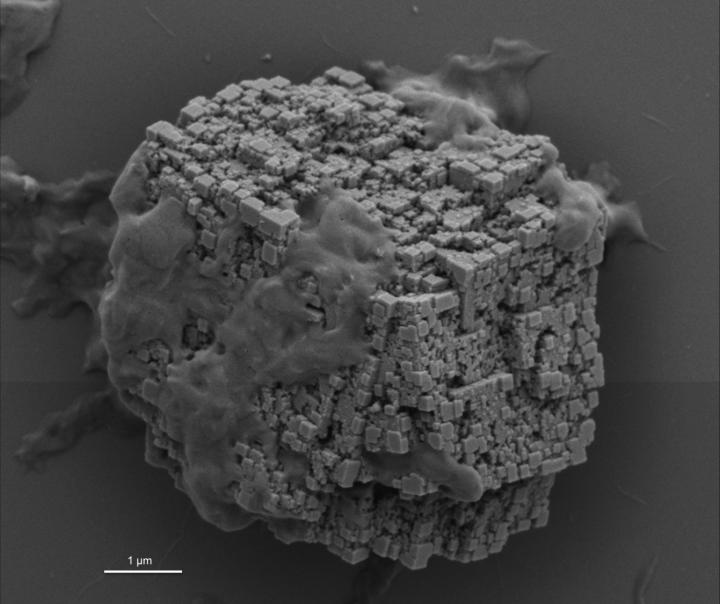
This is a scanning electron microscopy image of a calcite crystal generated in the presence of the sea urchin protein rSpSM50 on a silicon wafer showing organized nanotexturing on exposed surfaces. Credit: NYU Dentistry: Evans
Calcium carbonate, or CaCO3, comprises more than 4% of the earth's crust. Its most common natural forms are chalk, limestone, and marble, produced by the sedimentation of the shells of small fossilized snails, shellfish, and coral over millions of years.
New York University College of Dentistry (NYU Dentistry) researchers are studying how nature creates three-dimensional CaCO3 inorganic/organic based materials to form seashells, invertebrate exoskeletons, and vertebrate bone, dentine, and enamel.
John Evans, DMD, PhD, a professor in NYU Dentistry's Department of Basic Science and Craniofacial Biology, oversees a research group focusing on the study of proteins that modulate the formation of biominerals, which in turn create new composite materials with unique properties, such as increased fracture and puncture resistances.
In a paper recently published in Biochemistry, Gaurav Jain, PhD, a postdoc in Dr. Evans's lab and coauthor of “A model sea urchin spicule matrix protein, rSpSM50, is a hydrogelator that modifies and organizes the mineralization process,” looked at how the CaCO3 matrix is organized inside a sea urchin spicule (See figure 1). At first, these spicules are nothing more than chalk, but when combined with sea urchin proteins, they form tiny stacks of “bricks,” creating a structure that provides some of the toughest defense against predators and harsh ocean conditions.
“Primary mesenchyme cells (PMCs) inside a sea urchin embryo deposits amorphous CaCO3 within the matrix of spicule proteins where these bricks are shaped into layers of calcium carbonate crystals,” notes Dr. Jain. “However, the functional and assembly capabilities of individual spicule matrix proteins aren't clear. We are currently investigating one such protein found inside the spicules of a sea urchin embryo to understand what makes these proteins such efficient 'brick organizers.'”
The researchers looked at SM50, one of the most abundant and well-studied proteins found inside these spicules. They found that a recombinant version of the SM50 protein, rSpSM50, is a highly aggregation-prone protein that forms tiny jelly-like structures called hydrogels in solution. These 'jellies' capture tiny mineral nanoparticles and organize them into crystalline 'bricks.' Moreover, rSpSM50 causes surface texturing and forms randomly interconnected porous channels within these crystals.
“What is unique about rSpSM50 is that it fosters the formation and organization of two different forms of calcium carbonate — calcite and vaterite within the 'jellies' themselves, inducing fracture resistance to the overall structure,” said Dr. Jain.
Researchers used a specific type of titration method that revealed the details about very early events in the spicule formation.
“rSpSM50 turns out to be a really important piece of the puzzle, as it slows the formation kinetics but neither stabilizes nor destabilizes the extremely tiny mineral particles that ultimately form these bricks,” says coauthor Martin Pendola, PhD.
CaCo3 has always been a man's favorite construction material to make primitive tools, musical instruments, and craftware since the beginning of civilization. In modern times, CaCO3 is the most widely used mineral in the paper, plastics, paints and coatings industries both as a filler — and due to its special white color — as a coating pigment.
“Our current research, funded by U.S. Department of Energy, will enable scientists to better understand the mineralization and assembly process crucial to spicule formation in sea urchin,” said Dr. Evans. “Our ultimate goal is to determine the molecular properties of these proteins that allow matrices to assemble, mineralize, and participate in the formation of naturally occurring organic/inorganic skeletal structures. The hope is that the comprehensive understanding of spicule proteins will enable the development of tunable fracture resistant materials that one day will find its use in developing lightweight 'armor' and 'sturdier' dental composites.”
###
Contributors:
**Gaurav Jain1; **Martin Pendola1; Yu-Chieh Huang2; Denis Gebauer2; and John Spencer Evans1
1. Laboratory for Chemical Physics, Center for Skeletal and Craniofacial Biology, New York University College of Dentistry, NY, NY, USA. 2. Department of Chemistry, Physical Chemistry, Universität Konstanz, Universitätstrasse 10, Konstanz D- 78457, Germany.
**Both authors contributed equally to this work
Acknowledgments: AFM imaging was conducted at the Molecular Cytology Core Facility, Memorial Sloan-Kettering Cancer Center, New York. Flow cytometry studies were conducted at the Department of Microbiology, Columbia University, New York. This report represents contribution number 83 from the Laboratory for Chemical Physics, New York University.
Funding sources: Portions of this research (recombinant protein synthesis, LM, flow cytometry, AFM, SEM, FIB, TEM) were supported by the Life Sciences Division, U.S. Army Research Office, under award W911NF-16-1-0262 (JSE). The potentiometric experiments were supported by the Zukunftskoleg of the University of Konstanz (DG).
About NYU College of Dentistry
Founded in 1865, New York University College of Dentistry is the third oldest and the largest dental school in the US, educating more than 8 percent of all dentists. NYU College of Dentistry has a significant global reach with a highly diverse student body. Visit http://dental.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Pinpointing hydrogen isotopes in titanium hydride nanofilms
Although it is the smallest and lightest atom, hydrogen can have a big impact by infiltrating other materials and affecting their properties, such as superconductivity and metal-insulator-transitions. Now, researchers from…

A new way of entangling light and sound
For a wide variety of emerging quantum technologies, such as secure quantum communications and quantum computing, quantum entanglement is a prerequisite. Scientists at the Max-Planck-Institute for the Science of Light…

Telescope for NASA’s Roman Mission complete, delivered to Goddard
NASA’s Nancy Grace Roman Space Telescope is one giant step closer to unlocking the mysteries of the universe. The mission has now received its final major delivery: the Optical Telescope…



