Seeing antibiotics in action inside a pathogenic bacterium
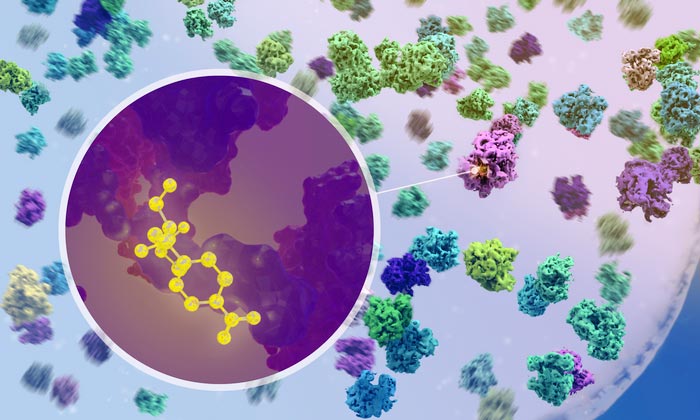
Researchers used cryo-electron tomography to visualise how antibiotics bind to ribosomes inside bacteria.
Credit: Isabel Romero Calvo/EMBL
New research by EMBL scientists shows at atomic detail how antibiotics affect the process of protein production inside bacteria.
Every living cell relies on proteins in order to function and the process of protein synthesis – translation – is critical for survival. Bacteria are no exception, with molecular machines involved in translation being one of the most common targets for antibiotics.
Now, for the first time, scientists led by Julia Mahamid’s group at EMBL Heidelberg have visualised at atomic detail how antibiotics affect the process of protein production inside bacterial cells. This study, published in Nature, also marks the first time when scientists have observed atomic-level structural changes in the active translation machinery directly inside a cell, rather than using isolated molecules in a test tube. Importantly, this approach allowed them to identify mechanisms that such machines use to “talk” to each other inside cells.
The study was carried out in collaboration with researchers from the Max-Planck-Institute for Biophysical Chemistry, Göttingen, the Wellcome Centre for Cell Biology, University of Edinburgh, and Technische Universität Berlin. The research also involved contributions from the Zimmermann-Kogadeeva and Bork groups at EMBL Heidelberg, who helped the researchers carry out bioinformatics analyses to observe what diversity in ribosomal proteins looks like across >4000 representative bacteria.
Minuscule bacteria and molecular machines
Mahamid and her team are experts in studying the bacterium Mycoplasma pneumoniae using a technique called cryo-electron tomography (cryo-ET). This tiny bacterium, which causes atypical pneumonia in humans, has a fully-functional protein synthesis machinery, despite being only about ten-thousandth of a millimetre in size.
“We chose Mycoplasma for our studies because they are among the smallest and most minimal living cells, and have been widely used as model cells in systems biology and synthetic biology studies,” said Liang Xue, postdoc in the Mahamid group and the first author of the study.
Cryo-ET allows researchers to take serial images of flash-frozen biological samples using an electron microscope and combine the resulting images to form a three-dimensional view of a cell – somewhat like a mini-MRI machine. “With large scale cryo-ET data from pristinely preserved cells, it is possible to capture high-resolution snapshots of different states of a molecular machine in action and combine them into a movie,” said Mahamid.
One of the most prominent structures when one looks at a cryo-ET image of a Mycoplasma cell are tiny dark blobs – these are the ribosomes. “The ribosome is one of the most ancient macromolecular machines, which may have existed even before the emergence of cells,” said Xue. Ribosomes are the primary molecular machines involved in protein translation, and they are present in all cells – from bacteria to human.
The Mahamid group’s approach allowed them to not only spot and count the ribosomes inside the bacteria, but also see their structure at atomic resolution. By studying a large number of ribosomes ‘frozen’ at different stages of their activity cycle, the scientists could decipher how the ribosome structure changes as it proceeds through the protein synthesis process. Not only this, but they could also localise the ribosomes in the three-dimensional space inside the cell, which allowed them to identify how the process of translation is organised spatially.
“Inside living cells, ribosomes function as highly interconnected systems, rather than individual molecular machines,” said Xue. “We revealed new features in ribosomes and the diverse translation reaction paths in cells.”
Antibiotics in action
Crucially, using cryo-ET, the researchers could observe what happens when antibiotics enter the cell and bind to ribosomes. For example, they could confirm that the two broad-spectrum antibiotics chloramphenicol and spectinomycin bind to different sites on the ribosome and disrupt different steps of the protein synthesis process. This had been predicted by studies on isolated ribosomes, but never before been observed in action inside an actual bacterial cell.
“It was very exciting when we were first able to see the drug molecule binding to a ribosome inside the cell,” said Xue. “But it was even more exciting when we found that the ribosome populations in antibiotic-treated cells are fundamentally reshaped – functionally, structurally, and spatially.”
The researchers observed that the interactions between ribosomes and other complexes in the cell changed in response to the drug, suggesting that an antibiotic can have an effect that reaches far beyond the specific complex it binds to. “On the one hand, this can help understand off-target effects of antibiotics, and may also help design combinations of antibiotics to increase their efficiency,” said Mahamid.
The Mahamid group continues to use the power of cryo-ET to study fundamental biological processes. “What we are able to do for this exquisitely simple model system is in principle applicable to much more complex models,” said Mahamid. “For example, in our group we study the interaction between viruses and their human cell host, the organisation of human pluripotent stem cells and the functioning of their ribosomes, and even large multicellular 3D organoids that our collaborators and we grow from cells taken directly from cancer patients.”
Journal: Nature
DOI: 10.1038/s41586-022-05255-2
Subject of Research: Cells
Article Title: Visualizing translation dynamics at atomic detail inside a bacterial cell.
Article Publication Date: 28-Sep-2022
Media Contact
Lisa Vollmar
European Molecular Biology Laboratory
lisa.vollmar@embl.org
Office: 15140088871
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



