Sentinels of the Genome
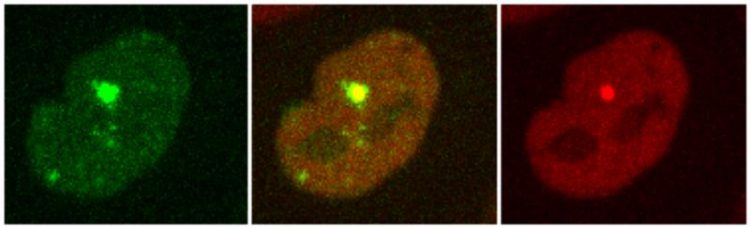
Recruitment of two proteins to the sites of DNA damage generated by laser micro-irradiation. Stoynov/ IMB-BAS
Throughout life, DNA is constantly being damaged by environmental and intrinsic factors and must be promptly repaired to prevent mutations, genomic instability, and cancer. Different types of damages are repaired by numerous proteins organized into damage-specific pathways.
The proteins from different pathways must be spatially and temporally coordinated in order to efficiently repair complex DNA damages. How this is achieved by the cell, is still poorly understood, due to the complexity and rapid dynamics of the process.
This question is particularly important since many anticancer drugs either damage DNA or target DNA repair proteins. A systematic study of the impact of such drugs on the overall coordination of the repair process could deliver new insights into their mechanisms of action, prompt new applications or suggest possible side effects.
An international team of researchers from the Institute of Molecular Biology at the Bulgarian Academy of Sciences (IMB-BAS), Sofia University, the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG), the Biotechnology Center (BIOTEC) and the Medical Faculty, both at the TU Dresden, and the Department of Mathematics of University of Pennsylvania built a high resolution, quantitative model of the dynamics of arrival and departure of 70 key DNA repair proteins at sites of complex DNA damage.
The researchers present their findings in the current issue of Molecular Cell. Combining the proteins based on their times of arrival, highlighted unexpected temporal aspects of complex DNA damage repair. The researchers could show that the proteins, which synthesize new stretches of DNA as part of the repair process, arrive at different times:
The proteins, which synthesize DNA without errors (error-free) are recruited within thirty seconds, while proteins performing imprecise DNA synthesis (error-prone) are recruited a minute later. The mechanism responsible for the delay in the error-prone synthesis, which is uncovered in this study, provides an opportunity for a precise repair of complex DNA damages.
The study also reveals that treatment with BMN673 (Talazoparib), a promising anticancer drug, dramatically changes the timescale of recruitment of DNA repair proteins at sites of complex damage. Notably, BMN673 delays the arrival of the error-free DNA synthesis machinery, which is loaded simultaneously with the error-prone repair proteins. The rearrangement in the order of the recruitment or removal of repair proteins as a result of BMN673 treatment, could affect the outcome of DNA repair and have a significant role for the anticancer activity of the drug.
The lead investigator Stoyno Stoynov from the IMB-BAS and former visiting scientist at the MPI-CBG and Alexander von Humboldt fellow at TU Dresden, concludes: ”This study generated a comprehensive kinetics-based resource which proved to be a powerful tool for investigating the interplay and coordination between DNA repair pathways. Even more importantly, it can serve as a platform for systematic evaluation of the effects of anticancer drugs targeting the DNA repair process.”
Aleksandrov, Radoslav et al. Molecular Cell, Volume 69 , Issue 6 , 1046 – 1061.e5
https://doi.org/10.1016/j.molcel.2018.02.016
About the MPI-CBG
The Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) is one of 84 institutes of the Max Planck Society, an independent, non-profit organization in Germany. 500 curiosity-driven scientists from over 50 countries ask: How do cells form tissues? The basic research programs of the MPI-CBG span multiple scales of magnitude, from molecular assemblies to organelles, cells, tissues, organs, and organisms.
About the IMB-BAS
The Institute of Molecular Biology is one of 42 institutes of the Bulgarian Academy of Sciences. IMB-BAS is the leading Bulgarian research institution in the area of molecular and cellular biology and biochemistry.
About the BIOTEC
The Biotechnology Center (BIOTEC) was founded in 2000 as a central scientific unit of the TU Dresden with the goal of combining modern approaches in molecular and cell biology with the traditionally strong engineering in Dresden. Since 2016 the BIOTEC is part of the central scientific unit “Center for Molecular and Cellular Bioengineering” (CMCB) of the TU Dresden.
The BIOTEC focuses on cell biology, biological physics, and bioinformatics.
Media Contact
More Information:
https://www.mpi-cbg.de/de/home/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



