Separating methane and CO2 will become more efficient
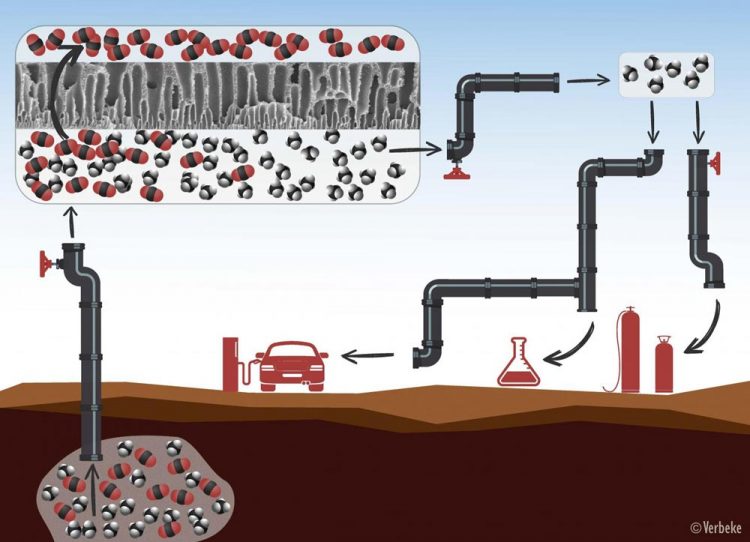
Natural gas or biogas always needs to be purified before use. First, the methane molecules (in black and white) are separated from the CO2 molecules (in red and black) by means of membranes with tiny pores through which only the CO2 can pass. After the purification process, the methane can be used as fuel, for heating, or for the production of chemicals. Credit: KU Leuven - Verbeke
When it comes to extracting natural gas or producing biogas, it's all about the methane. But methane is never found in its pure form. Natural gas, for instance, always contains quite a bit of carbon dioxide (the greenhouse gas CO2), sometimes up to 50 percent.
To purify the methane – or, in other words, remove the CO2 – the industry often uses membranes. These membranes function as molecular sieves that separate the methane and the CO2. The methane can then be used as a source of energy for heating, for the production of chemicals, or as fuel, while the CO2 can be reused as a building block for renewable fuels and chemicals.
Existing membranes still need to be improved for effective CO2 separations, says Professor Ivo Vankelecom from the KU Leuven Faculty of Bioscience Engineering. “An effective membrane only allows the CO2 to pass through, and as much of it as possible.
The commercially available membranes come with a trade-off between selectivity and permeability: they are either highly selective or highly permeable. Another important problem is the fact that the membranes plasticise if the gas mixture contains too much CO2. This makes them less efficient: almost everything can pass through them, so that the separation of methane and CO2 fails.”
The best available membranes consist of a polymeric matrix with a filler in it, for instance a metal-organic framework (MOF). This MOF filler has nanoscale pores. The new study has shown that the characteristics of such a membrane improve significantly with a heat treatment above 160 degrees Celsius during the production process.
“You get more crosslinks in the polymeric matrix: the net densifies, so to speak, and that in itself already improves the membrane performance, because it can no longer plasticise. At these temperatures, the structure of the MOF – the filler – changes, and it becomes more selective. Finally, the high-temperature treatment also improves polymer-filler adhesion: the gas mixture can no longer escape through little holes at the filler-polymer interface.” This gives the new membrane the highest selectivity ever reported, while preventing plasticisation when the concentration of CO2 is high. “If you start off with a 50/50 CO2/methane mixture, this membrane gives you 164 times more CO2 than methane after permeation through the membrane,” Dr Lik Hong Wee explains. “These are the best results ever reported in scientific literature.” This study is a collaboration between KU Leuven (Professor Ivo Vankelecom and Dr Lik Hong Wee from the Faculty of Bioscience Engineering / Centre for Surface Chemistry and Catalysis) and UAntwerp (EMAT unit led by Professor Sara Bals). Project website: http://www.
Media Contact
Professor Ivo Vankelecom
ivo.vankelecom@kuleuven.be
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative vortex beam technology
…unleashes ultra-secure, high-capacity data transmission. Scientists have developed a breakthrough optical technology that could dramatically enhance the capacity and security of data transmission (Fig. 1). By utilizing a new type…

Tiny dancers: Scientists synchronise bacterial motion
Researchers at TU Delft have discovered that E. coli bacteria can synchronise their movements, creating order in seemingly random biological systems. By trapping individual bacteria in micro-engineered circular cavities and…

Primary investigation on ram-rotor detonation engine
Detonation is a supersonic combustion wave, characterized by a shock wave driven by the energy release from closely coupled chemical reactions. It is a typical form of pressure gain combustion,…



