Study reveals how ribosomes are assembled in human cells
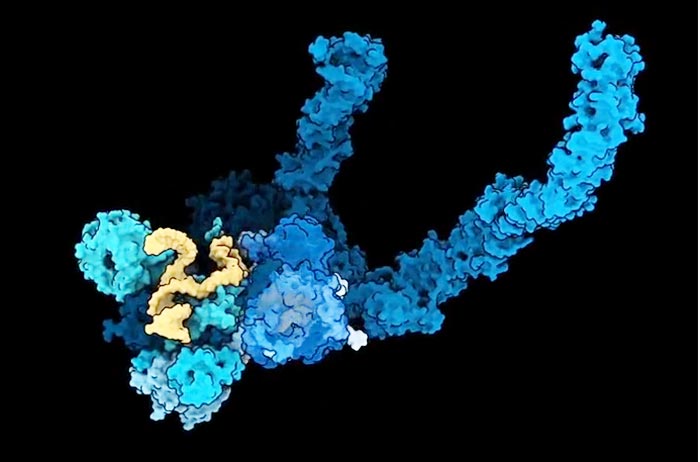
Ribosome assembly is an intricate process. The early stages of the assembly, shown above, takes place inside the nucleolus, a structure deep inside the cell’s nucleus.
Credit: Arnaud Vanden Broeck
All cells need ribosomes to make the proteins necessary for life. These multi-component molecular machines build complex proteins by stitching building blocks together according to instructions encoded in the cell’s messenger RNAs. But ribosomes are themselves composed of small and large subunits, each of which is made up of ribosomal proteins and RNA. Before they can manufacture proteins, these subunits must be manufactured themselves.
In a new study, scientists in the lab of Sebastian Klinge provide the most detailed view of how human small ribosomal subunits are put together by capturing their 3D portraits at three different stages of the assembly process. The findings are published in Science.
“The assembly of a ribosome is like an origami,” says Klinge, associate professor and head of the Laboratory of Protein and Nucleic Acid Chemistry. “Segments of RNA and other proteins have to be accurately folded in precise steps. The fundamental problem we are trying to understand is how proteins known as assembly factors work in concert to control each step of the assembly.”
For the study, the researchers developed a human gene-editing platform to tag ribosome assembly factors and established a novel biochemical procedure to overcome the hurdle of extracting the pre-ribosomal particles from the nucleolus, a structure inside the cell’s nucleus. These particles were then imaged using cryo-electron microscopy, revealing their structure at near atomic resolution.
The findings detail how some 70 assembly factors come together to create a scaffolding for the construction of the small subunit, and to guide each step of its maturation. Once their job is done, the assembly factors break apart, liberating the mature small subunit they held inside.
The three stages captured in the study provide a better understanding of the key molecular mechanisms that bring about the formation of the small subunit. The findings also provide new insights into rare human diseases that result from mutations in ribosomal proteins or assembly factors during the assembly of ribosomes.
Journal: Science
DOI: 10.1126/science.abj5338
Article Title: Nucleolar maturation of the human small subunit processome
Media Contact
Katherine Fenz
Rockefeller University
kfenz@rockefeller.edu
Office: 212-327-7913
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



