The fatal role of T cells in COVID-19
The fatal role of T-cells in COVID-19
(c) BIH/Birgit Sawitzki
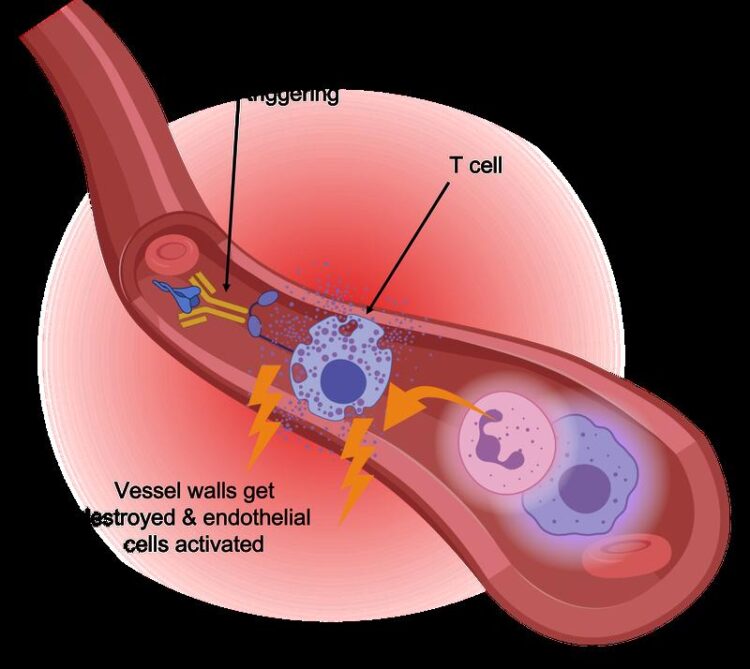
Scientists from the Berlin Institute of Health at Charité (BIH), together with colleagues from Charité – Universitätsmedizin Berlin and the university hospitals in Bonn and Aachen, have found a type of immune cells that is particularly active in severely ill COVID-19 patients. The CD16 positive T cells have an increased cytotoxic effect, especially on the inner cell layer of blood vessels. Their presence, along with complement system factors, is associated with a highly fatal outcome of the disease. The scientists have just published their findings in the scientific journal Cell.
It is now virtually certain that a dysfunctional immune system plays a key role in severe COVID-19: Overactive immune cells attack and destroy the body’s own tissue, even if the actual viral infection has already been contained or even overcome. Professor Birgit Sawitzki, head of the Translational Immunology Department at the BIH, is particularly interested in the role of T cells in SARS-CoV-2 infection. “T cells are the conductors of the whole orchestra of immune cells and signaling molecules,” she explains. “T helper cells make it possible to develop a targeted defense using tailored antibodies, T killer cells specifically destroy infected or malignant cells in the body, and regulatory T cells make sure everything stays in sync. Unfortunately, certain T cells are responsible for a particularly severe course of COVID-19.”
Safety mechanism is put out of action
“We knew that SARS-CoV-2 infection causes T cells to proliferate that specifically recognize and respond to the spike protein – even in patients who develop a severe disease course. So the severe course of the disease is apparently not due to the immune response being too weak,” explains Philipp Georg, the co-lead author of the current paper and a PhD student with Prof. Leif Erik Sander of Charité’s Department of Infectious Diseases and Respiratory Medicine. Sander played a key role in the research and led the clinical part of the study, among other contributions. To find out what role T cells play in COVID-19, the scientists used single-cell analyses to examine the blood of patients with mild or severe COVID-19 disease and compared it with the blood of healthy subjects and of patients with other viral infections. They discovered T cells carrying the CD16 molecule on their surface in individuals with severe COVID-19. “This was a surprise to the immunology community,” Sawitzki reports, “because CD16 is actually expected on cells of the innate immune system like natural killer cells or monocytes, but not on T cells that belong to the acquired or specific immune system.”
CD16 helps cells of the innate immune system recognize and kill virus-infected cells in the body. The molecule detects antibodies bound to virus-infected cells and then stimulates immune cells to release cytolytic enzymes, which destroy the virus-infected cells. Yet T cells are not in need of such help. “T cells identify virus-infected cells through their T cell receptor, which specifically binds to presented viral components, thereby stimulating the T cell to kill the target cell. Additional activation by CD16, independent of the T cell receptor, can significantly increase the destructive function of T cells,” Sawitzki explains. “This is dangerous because T cells actually have a built-in safety mechanism: They use their T cell receptors to track down foreign protein substances, so their activity is directed only against infected or altered body cells. Activation via CD16 overrides this safety mechanism, enabling non-infected vascular cells to be attacked.”
The complement system is also involved
The researchers observed in laboratory experiments that upon contact with antibodies, the CD16 positive T cells released cytotoxic molecules and damaged pulmonary vascular cells. In collaboration with researchers from Aachen, they also discovered CD16-positive T cells in the lungs of deceased COVID-19 patients. “This confirmed our suspicion that these cells play a fatal role in the course of COVID-19 disease,” explains Rosario Astaburuaga Garcia, one of the lead authors and a PhD student with Professor Nils Blüthgen of the Institute of Pathology at Charité and of the Integrative Research Institute for the Life Sciences at Humboldt-Universität zu Berlin. “We were surprised by the fact that activated CD16 positive T cells are not found in individuals with other severe infections such as HIV or hepatitis.”
While searching for the origin of CD16 positive T cells, the scientists came across the so-called complement system: This encompasses over 30 proteins that are dissolved in the blood plasma to help fend off microorganisms. They are activated in the course of the immune response by various mechanisms, such as by bound antibodies, and lead to the elimination of infected cells. “We have found that certain components of this system are abundantly produced in patients with a severe course of COVID-19 and contribute to the emergence of CD16 positive T cells. Here we seem to have discovered an important new link,” Sawitzki suspects. “If this link is confirmed, inhibiting the complement system could potentially help reduce severe courses to a minimum.” This is precisely the avenue that the scientists now want to explore further. The research has been supported by the “COVIM” AP4 program of the German Network of University Medicine (NUM), among others.
Original publication: P. Georg, R. Astaburuaga-Garcia,…..and B. Sawitzki: “Complement activation induces excessive T cell cytotoxicity in severe COVID-19”; Cell, DOI: 10.1016/j.cell.2021.12.040
About the Berlin Institute of Health at Charité (BIH)
The mission of the Berlin Institute of Health at Charité (BIH) is medical translation: transferring biomedical research findings into novel approaches to personalized prediction, prevention, diagnostics and therapies and, conversely, using clinical observations to develop new research ideas. The aim is to deliver relevant medical benefits to patients and the population at large. As the translational research unit within Charité, the BIH is also committed to establishing a comprehensive translational ecosystem – one that places emphasis on a system-wide understanding of health and disease and that promotes change in the biomedical translational research culture. The BIH was founded in 2013 and is funded 90 percent by the Federal Ministry of Education and Research (BMBF) and 10 percent by the State of Berlin. The founding institutions, Charité – Universitätsmedizin Berlin and Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC), were independent member entities within the BIH until 2020. Since 2021 the BIH has been integrated into Charité as its so-called third pillar. The MDC is now the Privileged Partner of the BIH.
Contact
Dr. Stefanie Seltmann
Head of Communications
Berlin Institute of Health at Charité (BIH)
+49 (0)30 450 543019
stefanie.seltmann@bih-charite.de
www.bihealth.org
Wissenschaftliche Ansprechpartner:
Prof. Birgit Sawitzki, BIH
Originalpublikation:
P. Georg, R. Astaburuaga-Garcia,…..and B. Sawitzki: “Complement activation induces excessive T cell cytotoxicity in severe COVID-19”; Cell, DOI: 10.1016/j.cell.2021.12.040
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



