Ultrafast lasers can protect a DNA building block from light-induced dissociation
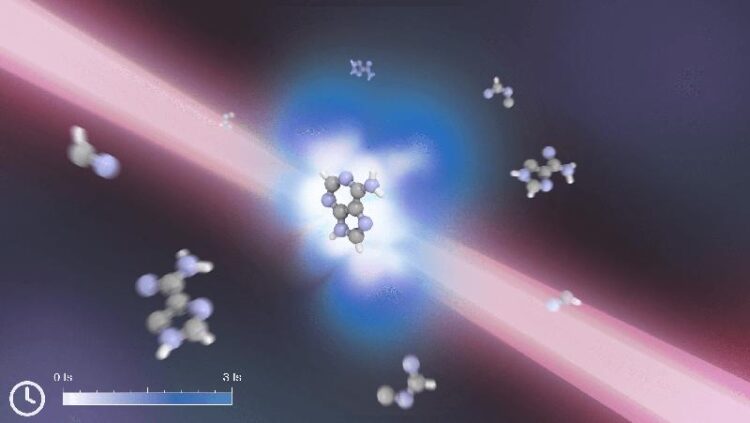
Wird das Adenin-Molekül durch VUV-Strahlung ionisiert, kommt es zur Aufspaltung. Durch richtiges Timing eines zweiten Infrarot-Laserpulses und die Ausnutzung der Ladungswanderung kann das Molekül jedoch durch eine zweite Ionisation stabilisiert werden.
U. de Giovannini, MPSD
An international research team – with a key contribution by the MPSD and led by DESY researcher Francesca Calegari – has demonstrated that ultrashort laser pulses can be used to protect one of the DNA building blocks against destruction induced by vacuum ultraviolet (VUV) radiation.
The research group unveiled that a second laser flash in the infrared spectrum, timed shortly (only a few millionths of billions of a second) after the first VUV flash, prevented the adenine molecule from disintegrating, therefore stabilising it. The group presents its work in the journal Communications Chemistry, by the Nature publishing group.
High energy radiation can cause irreparable damage to our own biological molecules – such as the DNA – leading to mutations and potentially cell death. Damage often occurs as a consequence of molecular ionization, which leads to the fragmentation of the DNA subunits. So far, protection against radiation damage has hardly been achieved because the photo-induced dissociation process could not be stopped. In their experiments, Francesca Calegari´s research group and collaborators have discovered that it is indeed possible to protect the molecule by taking advantage of mechanisms that take place on extremely fast time scales.
Calegari’s attosecond research group is based at the Center for Free-Electron Laser Science (CFEL), which is jointly operated by DESY, the University of Hamburg and the Max Planck Society. Its members work with extremely short light flashes in the attosecond range in order to depict and control electron movements in complex molecules. In their recent experiments, the researchers exposed molecules of the DNA building block adenine to an intense VUV flash for a time of attoseconds (10-18 seconds), which normally causes the destruction of the molecule.
By exposing the molecule to an infrared light flash only two femtoseconds (10-15 seconds) later, they found that the molecule stabilized itself by emitting an electron and its dissociation was stopped. Instead, a doubly ionised but otherwise intact adenine molecule remained. Overall, the group was thus able to save about one per cent of the molecules from destruction. The key mechanism behind this stabilization has been found to be “electron charge migration”: a purely electronic process involving an ultrafast charge inflation away from the molecule.
“In our experiments we have entered the attosecond range and were thus able to show – for the first time – that with our technology it is possible to take advantage of electron movements to change the fate of a molecular reaction,” explains first author Erik Månsson from DESY. “Until now, there have been only speculations about this possibility and the usual assumption was that controlling the movements of the atomic nuclei in the molecules was key for the molecular stabilisation.” This study has been conducted on time scales in which the atoms in the molecule can be considered as frozen, therefore unveiling a purely electronic mechanism so far unexplored for the building blocks of DNA.
The MPSD’s scientists provided the microscopic understanding of the experimental measurements by developing theoretical methods aimed at simulating the ultrafast correlated electron dynamics. The method is both efficient and applicable to realistic systems.
Co-author Simone Latini, a postdoc at the MPSD, is thrilled with the results: “It was fascinating to witness the advances in the experimental techniques for resolving electron dynamics on such short time scales,“ he says. „It’s also amazing to be part of the development of theoretical tools which allow us to describe realistic biomolecular systems that are so relevant for our life.”
The new findings are an important step towards understanding the fastest mechanisms activated by the interaction of biomolecules with light and their role in photoprotection. “The demonstration of an ultrafast stabilization protocol for a DNA subunit, by interrogating the system before the nuclear motion takes place, can substantially improve our ability of controlling ionisation damage effects – with interesting perspectives for protecting molecules against light,” explains Calegari.
Wissenschaftliche Ansprechpartner:
Erik Mansson, lead author: erik.mansson@desy.de
Simone Latini, co-author: simone.latini@mpsd.mpg.de
Originalpublikation:
https://www.nature.com/articles/s42004-021-00510-5
Weitere Informationen:
https://www.mpsd.mpg.de/550620/2021-05-manssonlatini-dna?c=17189
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



