Uncovering new details of the brain’s first line of defense
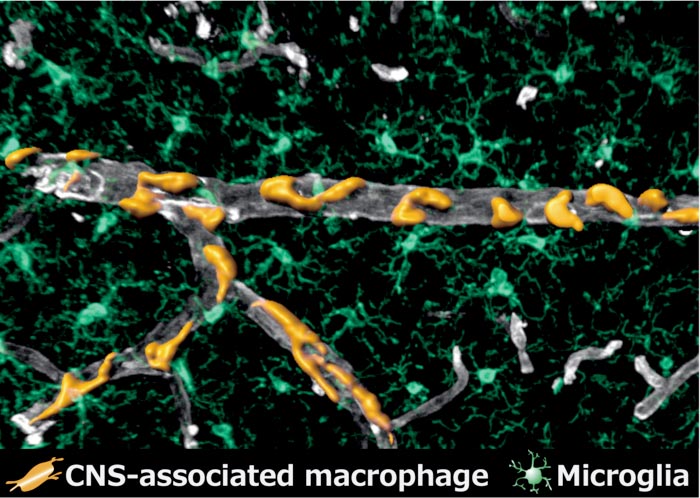
Perivascular macrophages (in orange) surrounding a vein in the brain (in grey). Microglia (in green) occupy the surrounding space as the other cells of the brain's immune system.
Credit: Kyushu University/Takahiro Masuda
Researchers report in unprecedented detail how some of the brain’s immune cells develop, and how to distinguish them from other cells.
Thanks to over a century of modern neuroscience, we have made significant strides in our understanding of the brain. Nonetheless, we have only just begun to scratch the surface of how this amazingly complex organ works.
Digging deeper into this perplexing puzzle, researchers from Kyushu University’s Faculty of Pharmaceutical Sciences have now analyzed in unprecedented detail the development and genetic profile of a set of cells that construct the brain’s immune system.
Their new insights, published in the journal Nature, could pave the way for better understanding the origins and mechanisms behind leading brain-related pathologies such as Alzheimer’s disease and multiple sclerosis.
“Many people are familiar with how neurons connect together to send signals across the brain, but there are also blood vessels that supply the brain with oxygen, and glial cells that act as the brain’s support network and immune system,” explains Takahiro Masuda, who led the study. “In fact, even the most generous estimates suggest only about half of the cells in our brains are neurons, so studying the other cells is just as vital for uncovering how the brain works.”
With this in mind, the research team has been focusing on a series of cells called ‘central nervous system associated macrophages’ a type of immune cells that protect the brain from infection. These macrophages are thought to be involved in almost all known neurodegenerative diseases due to their critical role as the immune cells of the brain.
Over the years, research has shown that many different kinds of these cells exist. For this study, the team was particularly interested in the macrophages surrounding blood vessels and those located in the meninges—the layers that surround the brain—known as ‘perivascular macrophages’ and ‘meningeal macrophages,’ respectively.
“Until now, these cells were not distinguished from other immune cells, and how and where these critical cells develop was significantly understudied,” continues Masuda. “So, we investigated fundamental characteristics of these cells, such as how to distinguish them from other cells in the brain, their exact locations, how they develop, what kind of genes they express, and how they interact with other cells.”
These macrophages, along with the brain’s other immune cells called microglia, originate from outside the embryo in an area known as the ‘yolk sac.’ As the organism develops, cells migrate from the yolk sac into the brain. Using a technique called ‘fate-mapping,’ the team precisely traced where these cells ended up and discerned what leads them to become perivascular macrophages and meningeal macrophages.
“We found that meningeal macrophages develop in the same way as other microglia and are formed during gestation. Perivascular macrophages, on the other hand, actually begin to form after birth, and originate from the meningeal macrophages. This was very unexpected,” states Masuda.
Through their research, the team was also able to identify the specific genes that lead to the generation of meningeal and perivascular macrophages.
“Identification of these genes will finally allow us to distinguish meningeal and perivascular macrophages from other microglia,” explains Masuda. “Now that we can study them individually, we can get a clearer picture of their functions.”
These findings are expected to open new avenues of understanding on the role of these cells in the brain.
“Now that we know they are distinct, the next step is to figure out their functions. As their mechanisms are revealed, we hope to understand their role in pathologies like Alzheimer’s, autism spectrum disorder, and multiple sclerosis,” concludes Masuda.
For more information about this research see “Specification of CNS macrophage subsets occurs postnatally in defined niches,” Takahiro Masuda, Lukas Amann, Gianni Monaco, Roman Sankowski, and Marco Prinz, et al., Nature (2022). https://doi.org/10.1038/s41586-022-04596-2
About Kyushu University
Kyushu University is one of Japan’s leading research-oriented institutes of higher education since its founding in 1911. Home to around 19,000 students and 8,000 faculty and staff, Kyushu U’s world-class research centers cover a wide range of study areas and research fields, from the humanities and arts to engineering and medical sciences. Its multiple campuses—including the largest in Japan—are located around Fukuoka City, a coastal metropolis on the southwestern Japanese island of Kyushu that is frequently ranked among the world’s most livable cities and historically known as a gateway to Asia.
Journal: Nature
DOI: 10.1038/s41586-022-04596-2
Method of Research: Experimental study
Subject of Research: Animals
Article Title: Specification of CNS macrophage subsets occurs postnatally in defined niches
Article Publication Date: 20-Apr-2022
Media Contact
Raymond Terhune
Kyushu University
sysintlkh@jimu.kyushu-u.ac.jp
Office: 92 802 2443
Original Source
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



