Why killer T cells lose energy inside of solid tumors
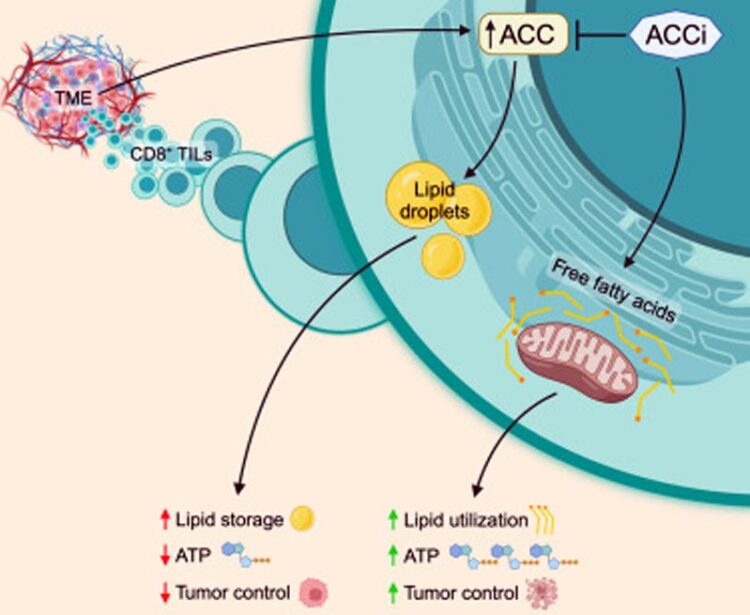
A graphical abstract showing how normal ACC expression causes lipid storage and inhibition of ACC leads to ATP production.
Credit: Hunt et al. 2024
T cells are often called “assassins” or “killers” because they can orchestrate and carry out missions to hunt down bacteria, viruses, and cancer cells throughout the body. Mighty as they may be, recent research has shown that once T cells infiltrate the environment of a solid tumor, they lose the energy needed to combat the cancer.
A research team led by Jessica Thaxton, PhD, MsCR, associate professor of cell biology and physiology and co-leader of the Cancer Cell Biology Program at the UNC Lineberger Comprehensive Cancer Center, aimed to understand why T cells do not sustain energy in tumors. Using their expertise in tumor immunity and metabolism, the Thaxton Lab, led by the Katie Hurst, MPH, and 4th year graduate student Ellie Hunt, found that a metabolic enzyme called Acetyl-CoA Carboxylase (ACC) causes T cells to store fat rather than burning fat for energy.
“Our discovery fills a long-standing gap in knowledge regarding why T cells in solid tumors don’t appropriately generate energy,” said Thaxton. “We inhibited the expression of ACC in mouse cancer models, and we observed that T cells were able to persist much better in solid tumors.”
The new findings and immunotherapeutic strategies, which were published in Cell Metabolism, could be used to make multiple types of T-cell therapies more effective for patients, possibly encompassing both checkpoint and chimeric antigen receptor (CAR) T-cell therapies.
In the field of cancer immunotherapy, it has long been known that T cells are not able to create their cellular energy, called adenosine triphosphate or ATP, when they are inside of a solid tumor.
In 2019, Thaxton’s lab studied a T cell with optimal antitumor function. In a publication in Cancer Immunology Research, Hurst and Thaxton used a proteomics screen to identify enzymes associated with the optimal antitumor metabolism of these T cells. Through this screen, the two discovered that ACC expression may limit the ability of T cells to make ATP in tumors. ACC, a key molecule that is involved in many metabolic pathways, blocks cells from breaking down fat and using it as fuel for energy in mitochondria.
“Acetyl-CoA carboxylase can drive the balance between storing lipids versus breaking down those lipids and feeding them into the citric acid cycle for energy,” said Thaxton. “If ACC is flipped ‘on’, cells generally store lipid. If ACC is ‘off’, cells tend to use the lipid in their mitochondria to make ATP.”
Using Hunt’s expertise in confocal imaging, the research team was able to observe lipid stores in T cells isolated from multiple types of cancers. The observation, as well as other experiments, confirmed the team’s hypothesis that T cells were storing lipids instead of breaking them down.
Thaxton’s team then used CRISPR Cas9-mediated gene deletion to see what would happen if they “deleted” ACC from the picture. There was a rapid reduction in the amount of lipid storage in T cells, and the team was able to visualize fat relocating to the mitochondria to be used to generate energy.
Thaxton now hypothesizes that T cells may need a “delicate balance” of lipids to persist in solid tumors with a certain amount of lipid dedicated to cancer cell assassination and low levels of fats being maintained in stores.
The latest findings could prove to be useful in enhancing chimeric antigen receptor (CAR) T-cell therapies. This cutting-edge technology takes T cells out of cancer patients, modifies them in the lab to hunt down tumor cells, and then re-infuses the cells to fight the patient’s cancer. Preliminary data from Thaxton’s lab demonstrates that even the manufactured T cells contain excess lipid stores.
The lab is starting to look in patient samples to understand how researchers can possibly flip the ACC metabolic switch directly in patient tumors, negating the need to take out and reinfuse cells back into the body. But researchers must first determine how this could affect other immune cell populations in the body, such as macrophages.
About UNC School of Medicine
The UNC School of Medicine (SOM) is the state’s largest medical school, graduating more than 180 new physicians each year. It is consistently ranked among the top medical schools in the US, including 5th overall for primary care by US News & World Report, and 6th for research among public universities. More than half of the school’s 1,700 faculty members served as principal investigators on active research awards in 2021. Two UNC SOM faculty members have earned Nobel Prize awards.
Journal: Cell Metabolism
DOI: 10.1016/j.cmet.2024.02.009
Article Title: Acetyl-CoA carboxylase obstructs CD8+ T cell lipid utilization in the tumor microenvironment
Article Publication Date: 14-Mar-2024
Media Contact
Kendall Daniels
University of North Carolina Health Care
kendall.daniels@unchealth.unc.edu
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



