How to make hydrogels more injectable
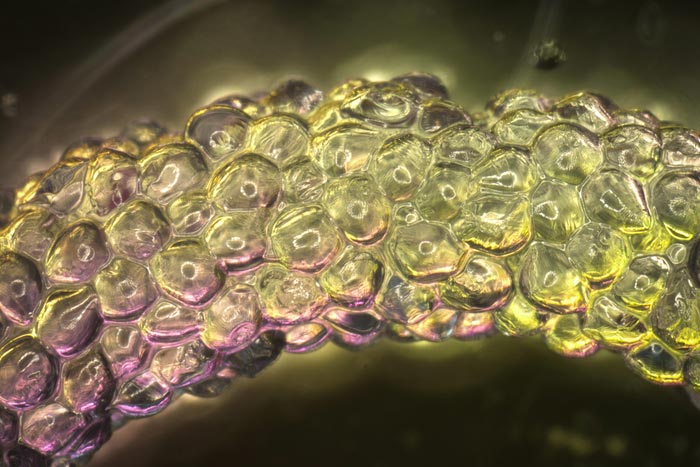
MIT and Harvard researchers have developed computational models that can predict the properties of materials made from squishy hydrogel blocks.
Credit: MIT
A new computational framework could help researchers design granular hydrogels to repair or replace diseased tissues.
Gel-like materials that can be injected into the body hold great potential to heal injured tissues or manufacture entirely new tissues. Many researchers are working to develop these hydrogels for biomedical uses, but so far very few have made it into the clinic.
To help guide in the development of such materials, which are made from microscale building blocks akin to squishy LEGOs, MIT and Harvard University researchers have created a set of computational models to predict the material’s structure, mechanical properties, and functional performance outcomes. The researchers hope that their new framework could make it easier to design materials that can be injected for different types of applications, which until now has been mainly a trial-and-error process.
“It’s really exciting from a material standpoint and from a clinical application standpoint,” says Ellen Roche, an associate professor of mechanical engineering, a member of the Institute for Medical Engineering and Science at MIT, and an author of the paper. “More broadly, it’s a nice example of taking lab-based data and synthesizing it into something usable that can give you predictive guidelines that could be applied to things beyond these hydrogels.”
Jennifer Lewis, the Hansjörg Wyss Professor of Biologically Inspired Engineering at Harvard, is the senior author of the study, which appears today in the journal Matter. Connor Verheyen, a graduate student in the Harvard-MIT Program in Health Sciences and Technology, is the lead author of the paper.
Material modeling
When individual hydrogel blocks are densely compacted together, they form a gel-like material known as a granular matrix. These materials can act as a solid or a liquid, depending on the conditions, which makes them good candidates for applications such as 3D-bioprinting engineered tissues. Once injected or implanted into the body, they could release drugs or help to regenerate injured tissue.
“These materials have a lot of flexibility and customizability, so there’s a lot of excitement about using them for biomedical applications,” Verheyen says.
While working in Lewis’ lab, Verheyen, who is co-advised by Lewis and Roche, began trying to figure out how to get these materials to be reliably injectable. This turned out to be a difficult task that required a lot of trial-and-error experimentation, by changing different features of the gels in hopes of optimizing their structure and mechanical behavior for injectability.
“That spurred the effort to take the empirical data, turn it into something that a machine could read and work with, and then ask it to build a predictive map that we could interrogate to help us understand what was going on and how to go to the next step,” he says.
To create their design framework, the researchers broke the assembly process down into several stages. They modeled each of these stages separately, using data from their own experiments, which were done under a variety of different conditions.
In the first stage, the model analyzed how bioblock properties are affected by the starting material of the blocks and how they are assembled. In the second stage, the bioblocks are packed together to form structures called “granular hydrogels.” Through their modeling, the researchers identified several factors that influence the injectability of the final gel, including the size and stiffness of the bioblocks, the viscosity of the interstitial fluid between the blocks, and the dimensions of the needle and syringe used to inject the gel.
Better injectability
Now that they have modeled the process from start to finish, the researchers can use their model to predict the best way to create a material with the traits they need for a particular application, instead of going through an extensive trial-and-error process for each new material.
“Our long-term goal was to get to the point where we had reliable and predictable injection properties, because that was something that we really struggled with in the lab — getting these materials to flow properly,” Verheyen says.
He and others in Roche’s lab now plan to use this modeling approach to try to develop materials that could be used for medical applications such as repairing heart defects or delivering drugs to the gastrointestinal tract.
The researchers have also made their models and the data they used to generate them available online for other labs to use.
“It’s all open source, and hopefully it will reduce the amount of frustration with issues that you might have reproducing something that happened in another lab, or even within one lab when you’re transferring knowledge from one person to another,” Roche says.
The research was funded by the Vannevar Bush Faculty Fellowship Program, the National Science Foundation, and a MathWorks Seed Fund grant.
Journal: Matter
DOI: 10.1016/j.matt.2023.01.011
Article Title: Integrated data-driven modeling and experimental optimization of granular hydrogel matrices
Article Publication Date: 31-Jan-2023
Media Contact
Sarah McDonnell
Massachusetts Institute of Technology
s_mcd@mit.edu
Office: 617-253-8923
Cell: 617-460-9583
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



