Membrane “nano-fasteners” key to next-generation fuel cells
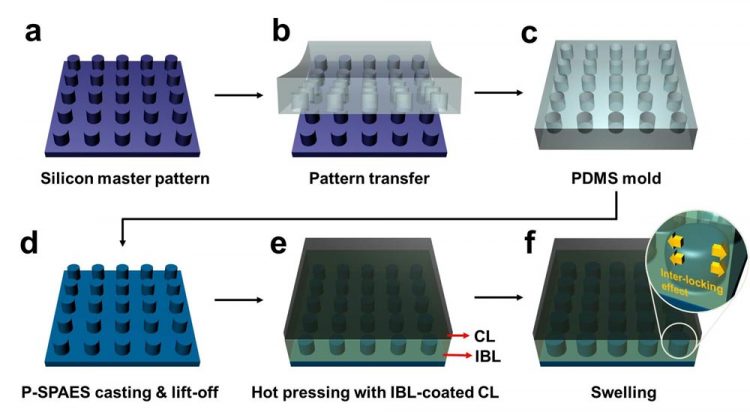
Schematic Diagram of the Fabrication of the Pillar P-SPAES Membrane and Its Working Principle of Interlocking Effects. Copyright : KAIST
The internal workings of fuel cells vary, but basically all types mix hydrogen and oxygen to produce a chemical reaction that delivers usable electricity and exhausts ordinary water as a by-product. One of the most efficient types is the proton exchange membrane (PEM) fuel cell, which operates at low enough temperatures to be used in homes and vehicles.
To generate electricity, PEM fuel cells rely on two chemical compartments separated by a permeable catalyst membrane. This membrane acts as an electrolyte; a negative electrode is bonded to one side of the membrane and a positive electrode is bonded to the other. The electrolyte membrane is often based on a polymer of perfluorosulfonic acid. Due to its high cost, however, a less expensive hydrocarbon-based electrolyte membrane has attracted interest in this technology sector.
Until now, the challenge in adopting such a hydrocarbon membrane has been that the interface between the electrode and hydrocarbon membrane is weak. This causes the membrane to delaminate relatively easily, falling apart and losing efficiency with use.
Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and his research team have developed a new fastening system that bonds the two materials mechanically rather than chemically. This opens the way to the development of fuel cell membranes that are less expensive, easier to manufacture, stronger and more efficient.
The researchers achieved this by moulding a pattern of tiny cylindrical pillars on the face of the hydrocarbon membrane. The pillars protrude into a softened skin of the electrode with heat. The mechanical bond sets and strengthens as the material cools and absorbs water. The pillar-patterned hydrocarbon membrane is cast using silicone moulds.
Professor Kim said, “This physically fastened bond is almost five times stronger and harder to separate than current bonds between the same layers.”
The new interlocking method also appears to offer a way to bond many types of hydrocarbon membranes that, until now, have been rejected because they couldn’t be fastened robustly. This would make hydrocarbon membranes practical for a number of applications beyond fuel cells such as rechargeable “redox flow” batteries.
The research team is now developing a stronger and more scalable interlocking interface for their nanoscale fasteners.
CONTACT:
Lan Yoon
The Korea Advanced Institute of Science and Technology (KAIST)
Tel. +82-42-350-2295
Fax. +82-42-350-2260
hlyoon@kaist.ac.kr
www.kaist.edu
Associated links
Original press release from KAIST
Media Contact
More Information:
http://www.researchsea.comAll latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Innovative vortex beam technology
…unleashes ultra-secure, high-capacity data transmission. Scientists have developed a breakthrough optical technology that could dramatically enhance the capacity and security of data transmission (Fig. 1). By utilizing a new type…

Tiny dancers: Scientists synchronise bacterial motion
Researchers at TU Delft have discovered that E. coli bacteria can synchronise their movements, creating order in seemingly random biological systems. By trapping individual bacteria in micro-engineered circular cavities and…

Primary investigation on ram-rotor detonation engine
Detonation is a supersonic combustion wave, characterized by a shock wave driven by the energy release from closely coupled chemical reactions. It is a typical form of pressure gain combustion,…



