2D images as the new tool for cancer prevention
2D images identify gastric cancer-prone mutations
This was clear when the new algorithm was tested on cells with mutated e-cadherin (a tumour suppressor protein) where it identified the mutations that produced malfunctioning e-cadherin that causes human diffuse gastric cancer (HDGC), effectively pinpointing the patients at risk of the disease.
The result is particularly important because HDGC is asymptomatic and, at the moment, its early detection is not reliable what means that the disease still has extremely high mortality. The new algorithm with its ability to identify patients at high risk, hopefully, will change that.
The study, out in the European Journal of Human Genetics, is a collaboration between João Sanches from the Institute for Systems and Robotics and Department of Bioengineering from the Instituto Superior Técnico at the Technical University of Lisbon that developed the algorithm, and the group of Raquel Seruca from the Department of Cancer Genetics of IPATIMUP – Institute of Molecular Pathology and Immunology at the University of Porto that works in cancer.
Gastric cancer is the 4th most common cause of cancer in the world, and hereditary diffuse gastric cancer (HDGC) makes up to 3% of all cases. Although not very frequent, the disease is hugely problematic for clinicians because of its high mortality, which is the result of several issues.
First, the fact that HDGC is caused by functional abnormalities/mutations in e-cadherin, an adhesion protein of epithelial cells (those covering the surfaces and inside of the body). E-cadherin holds epithelial cells together by lying across the cell membrane, one end attached to e-cadherins from neighbouring cells and the other to the skeleton of the epithelial cell.
When e-cadherin stops working, the cells become loose. In the case of HDGC, this means that there is no solid tumour, but, instead, a loose layer of cancerous cells, which easily move spreading the disease and making its control impossible unless detection occurs early. This is also why e-cadherin is known as an important tumour suppressor molecule.
The second problem when trying to control the disease is the difficulty of spotting HDGC early. In fact its initial symptoms are very non-specific (stomach acidity and burping for example), so easy to miss. And without clear symptomology, early detection relies on searching gastric cells for lack of e-cadherin on the membrane (a malfunctioning e-cadherin also leaves the membrane to go inside the cell to be destroyed) with results that are not always reliable.
As consequence there has been a major effort to improve HDGC detection, and it is here that Sanches’ algorithm steps in. This new method has many advantages – it is semi-automated what allows, on one hand, the human operators to resort to their experience to choose the more representative cells, and on the other to rely on the computer to normalise results from cells with very different sizes and shapes, and work even with very heterogeneous cell populations. Resorting to images assured that the cells were hardly altered. All of these secured that the results were accurate and representative.
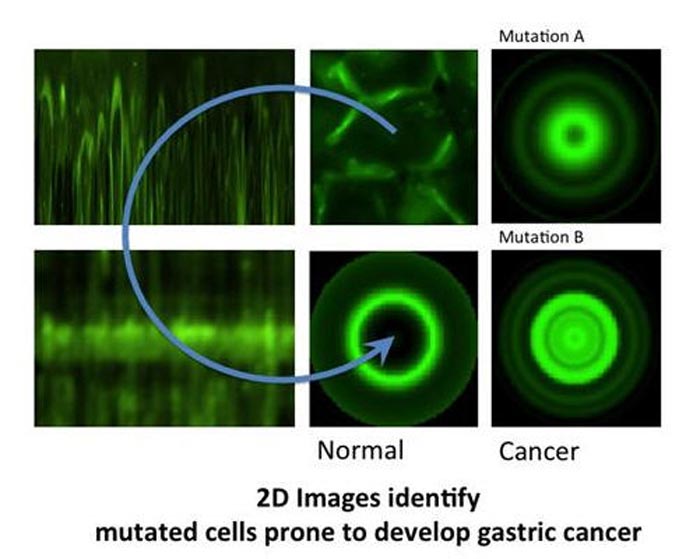
But how did it actually work? The software was designed to compute data from fluorescence images of the protein in a cell population, to generate “maps” of the protein distribution in that population. From these, it constructed 2D virtual images of a “typical cell” in that population, and it also calculated a new parameter called maximum mean ratio (MMR), which quantifies the sharpness of the protein fluorescence peak (so effectively measures the protein quantity). In HDGC experiments, a high MMR meant that e-cadherin has high expression by the membrane (as it should), and low levels inside of the cell
To see if this data could be used to improve the clinical management of HDGC, next, the researchers used the algorithm to compare cells with working e-cadherins, with those carrying mutations that made the protein non-functional/useless (so cancer prone). The idea was, that since normal e-cadherin is on the membrane and its malfunctioning form moves inside the cell to be destroyed, the new algorithm, by giving e-cadherin location, should be able to identify the individuals at risk of HDGC.
And in fact, after constructing 2D images of the two types of cells, the distinction was clear. While normal cells (or those with neutral/innocuous mutations) produced an image of a fluorescence circle with a clear centre (which represented the absence of protein inside the cell), those with malfunctioning e-cadherin (that can lead to HDGC) showed a full fluorescence circle (the fluorescence in the centre represented e-cadherin inside cell).
Additionally, these last cells had much lower MMR than normal, meaning less e-cadherin by the membrane and, in consequence, a weaker cell-to-cell adhesion what agreed with their propensity to cancer. “Our algorithm was not only able to pinpoint the protein location, but also to quantify it in each cellular compartment. While the MMR value gave us the protein dispersion.” – says Seruca
In conclusion, with a combination of 2D images and quantitative “maps” should now be much easier for researchers (and in the future clinicians) to quickly and reliably identify those individuals with mutations that lead e-cadherin to lose its function, and who, as result, are prone to develop HDGC, and who should be put under close monitoring for early signs of disease.
“Our algorithm can now be used as a complementary approach to evaluate the pathogenicity of E-cadherin.”- says Seruca – “Moreover, it can be applied to a wide range of proteins and, more importantly, to diseases characterized by aberrant protein expression or trafficking deregulation. “
And although the study has focused on HDGC, e-cadherin mutations are known to be involved in a variety of other cancers including breast, colorectal, thyroid and ovarian so these new results could also be applied to them.
http://www.nature.com/ejhg/journal/vaop/ncurrent/full/ejhg2014240a.html
Full bibliographic information
European Journal of Human Genetics (2014), 1–8
Quantification of mutant E-cadherin using bioimaging analysis of in situ fluorescence microscopy. A new approach to CDH1 missense variants
João Miguel Sanches, Joana Figueiredo, Martina Fonseca1, Cecília Durães, Soraia Melo, Sofia Esménio and Raquel Seruca*
Media Contact
All latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…



