Benefitting from Gentler Therapies and Diagnostics
If a patient is delivered to the clinic with pulmonary complications, the clinic has to decide which type of therapy is suitable. In addition to purely medical aspects other criteria also play an important role such as: the mental and physical stress on the patient due to the treatment, the time it takes to implement a measure and the overall economics of the procedure.
Risk of infection in the hospital
Non-invasive respiration is a form of therapy that is usually not only more pleasant for the patient but also reduces the risk of infection, and is therefore more economic. It is gradually being recognised that non-invasive respiration should be used wherever possible in day-to-day clinical use. This view is now also reflected in medical guidelines.1
The reason for this recommendation is that invasive respiration is accompanied by the risk of ventilator-associated pneumonia (VAP) – one of the most frequently occurring hospital-acquired infections in the US and in Europe.2
These complications not only impact on patients but also on the budget: infections acquired in US hospitals cost $29 million per year.3
Non-invasive applications in other areas
Non-invasive measures are generally becoming the focus of attention for preventing hospital-acquired infections. This is because each intervention, e.g. with needles or catheters, opens up a primary entrance point for infections. Furthermore, in a situation that is already stressful for patients, invasive procedures usually cause pain and anxiety. The same can be true for diagnostics and monitoring. However, whereas producing EKGs and monitoring oxygen saturation (SpO2 levels) and expiratory CO2 levels have been monitored non-invasively for a long time, it is now possible to continuously monitor arterial blood pressure as well.
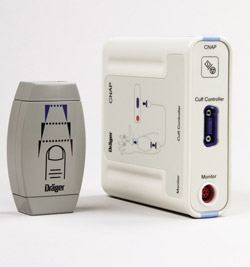
Dräger offers comprehensive solutions both for non-invasive respiration and monitoring. For example, the Carina respiration apparatus, in particular, fulfils the requirements for non-invasive respiration, but virtually all other Dräger respiration devices offer this option. The NovaStar face mask can be individually adapted to the patient’s facial contours and gel cushion ensures a tight fit, thereby minimizing leakage and skin lesions. Dräger’s Infinity CNAP SmartPod offers a method of measuring arterial blood pressure via a sensor sleeve that is placed over the patient’s finger. The Infinity etCO2 pod can be connected to the respiration tube system and measures the CO2 content of the exhaled air in the main and subsidiary flow.
Quick diagnostics procedure for alcohol and substance abuse
In addition, Dräger is also presenting two other devices at the Medica 2008 (in Dusseldorf) that offer non-invasive diagnostic procedures and are of particular interest to emergency rooms: the Dräger DrugTest 5000 and the Dräger AlcoTest 6810 med.
From a saliva sample, the Dräger DrugTest 5000 detects six different types of illegal drugs within minutes. The Dräger AlcoTest 6810 med checks the alcohol level of a patient’s breath. Both devices, which are already used for roadside checks, make it possible to quickly rule out alcohol or drug abuse and thus help reduce the number of cost- and time-intensive blood lab analyses.
1 For example in Germany, see Schönhofer, Bernd et al.: Nicht invasive Beatmung bei akuter respiratorischer Insuffizienz (Non-invasive respiration for acute respiratory insufficiency). In: Deutsches Ärzteblatt 2008; 105(24): 424-433. (Summary)
2 Prevention of hospital-acquired pneumonia – notification by the Commission for Hospital Hygiene and Infection Prevention at the Robert Koch Institute. In: Federal Health Bulletin – Health Research – Health Protection, 2000, 43:302-9.
3 Institute of Medicine, To Err is Human: Building a Safer Health System, National Academy
Press, 2000: Washington DC.
Dräger. Technology for Life®
Dräger is an international leader in the fields of medical and safety technology. Dräger products protect, support and save lives. Founded in 1889, in 2007 Dräger generated revenues of around EUR 1.8 billion. The Dräger Group employs around 10,000 people in more than 40 countries worldwide.
Contact for Trade Press
Christine Reimann
Tel: +49 451 882 1547
E-Mail: christine.reimann@draeger.com
Media Contact
More Information:
http://www.draeger.comAll latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



