Detecting pathogens faster and more accurately by melting DNA
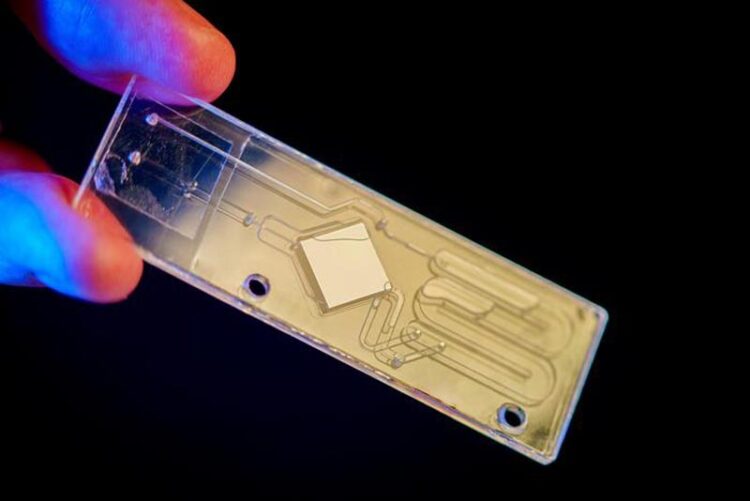
Researchers use this chip to analyze the microbes present in whole blood samples.
Credit: David Baillot/University of California San Diego
A new analysis method can detect pathogens in blood samples faster and more accurately than blood cultures, which are the current state of the art for infection diagnosis. The new method, called digital DNA melting analysis, can produce results in under six hours, whereas culture typically requires 15 hours to several days, depending on the pathogen.
Not only is this method faster than blood cultures, it’s also significantly less likely to generate false positives compared to other emerging DNA detection-based technologies such as Next Generation Sequencing.
Why does it matter?
It’s an experience most parents have had: you take your child to the doctor, because they’re running a fever, maybe coughing or sneezing. Your child has an infection, the doctor says, but it’s unclear if it’s bacterial or viral. Sometimes, the doctor will prescribe antibiotics “just in case.” Sometimes, they’ll order a blood draw to see if bacteria are present. Sometimes, the result will come back negative two to three days later, at which point you’ll be asked to keep giving the antibiotics to your child so they won’t start culturing antibiotic-resistant bacteria in their body.
This same scenario plays out in pediatric ICUs and emergency rooms, with higher stakes, when a child presents symptoms of sepsis. In this case, up to 30% of patients receive the wrong treatment, which actually puts them at higher risk of dying. With sepsis, speed is even more of the essence, since the mortality risk increases by 4% every hour that the infection goes undiagnosed or inaccurately treated.
Researchers conducted a pilot clinical study of blood samples from pediatric patients and showed that the results from their method exactly matched blood culture results in detecting sepsis. But their method detected pathogens 7.5 hours to about 3 days faster than clinical blood culture. The tests also go beyond a simple positive or negative result to quantify how much of the pathogen is present in samples.
The method relies on universal digital high-resolution DNA melting, where DNA is heated until it comes apart. Each sequence of DNA has a specific signature during melting. As the melting process is imaged and analyzed, machine learning algorithms determine which types of DNA are present in the samples and identify pathogens.
The research team presents their findings in the Feb. 21 issue of The Journal of Molecular Diagnostics.
“This is the first time this method has been tested on whole blood from patients suspected of having sepsis. So this study is a more realistic preview of how the technology could perform in real clinical scenarios,” said Stephanie Fraley, the paper’s senior author and a professor in the Shu Chien-Gene Lay Department of Bioengineering at the University of California San Diego.
An estimated one out of every five deaths worldwide is due to sepsis-related complications. And 41% of these deaths occur in children. Early detection is critical for sepsis survival, as mortality risk rises by 4% for every hour the infection goes undiagnosed or inaccurately treated.
Typically, physicians put sepsis patients on antibiotics while awaiting results from blood cultures. This can lead to antibiotic resistance down the line.
“The bottom line is, we’re not treating based on evidence,” Fraley said. “And the more we treat without evidence, the more we can cause unintended problems. Sometimes, we’re treating patients who have fungal or viral infections with antibacterials. This can cause antibiotic resistance and alter the patient’s microbiome in a significant way.”
How the method works
It all started with one milliliter of blood from each sample from 17 patients in the pilot clinical study. The samples were collected at the same time as samples for blood cultures from infants and toddlers.
Researchers perfected DNA isolation and machine learning methods to reduce or eliminate signals from human DNA compared to pathogen DNA in the samples.“Since human DNA significantly outnumbers pathogen DNA, this allows us to better detect the ‘needle in the haystack’ that is the pathogen,” Fraley said.
Mridu Sinha, one of Fraley’s former Ph.D. students and now CEO of Melio, the startup company they cofounded, optimized a machine learning algorithm to reliably detect the difference between melt curves from the pathogens and background noise. The algorithm matches the curves to a database of known DNA melt curves. It’s also able to detect curves created by organisms that are not in this database, which could show up in a sample if it contains rare or emerging pathogens.
The results not only matched exactly the results from blood cultures from the same blood samples; they also did not produce any false positives. By contrast, other types of tests relying on nucleic acid amplification and next-generation DNA sequencing databases will amplify any DNA present, leading to false positives. Often, DNA gets into the s ample from the environment, test tubes, reagents, skin and more. Sample contamination can cause issues with knowing how to interpret the test results.
“Our test has incorporated sample preparation processes, assay design techniques, and algorithms that ensure we only detect DNA from intact organisms, which is clinically relevant,” Sinha said.
Next steps include conducting a broader clinical study, as well as expanding the method to adult patients.
Fraley and Sinha licensed the technology from UC San Diego and cofounded startup Melio to commercialize their method.
“We want to give doctors the ability to treat their patients based not on aggregate data, but with precise, accurate individual data, enabling truly personalized medicine,” Fraley said.
What is DNA melting?
The DNA in the blood samples is heated causing it to melt at temperatures between 50 to 90 degrees Celsius–about 120 to 190 degrees Fahrenheit.
As the DNA double-helix melts, the bonds holding together the DNA strands break. Depending on the DNA’s sequence, the bonds have different strengths, and that changes the way the strands unwind from each other. This creates a unique sequence-dependent fingerprint, which researchers can detect using a special dye. The dye causes the unwinding process to give off fluorescent light, creating what researchers call a melting curve—a unique signature for each type of pathogen.
In the past, DNA melting has produced simple curves that were used primarily to confirm that a PCR reaction worked, but this new approach advances melting to generate complex melt curve signatures that are unique to gene sequences.
The work was funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award number R01AI134982 to S.I.F), a Burroughs Wellcome Fund Career Award at the Scientific Interface (award number 1012027 to S.I.F.), and UCSD Clinical Translational Research Institute and UCSD Accelerating Innovations to Market pilot grants.
Quote from Dr. Karen Mestan, Chief of the Division of Neonatology at Rady Children’s Hospital and the UC San Diego Department of Pediatrics:
“The findings of this study fill an important need in pediatrics, especially for critically ill infants and small children in which clinical signs of bacteremia are extremely difficult to decipher. In settings of early subclinical sepsis, and also overwhelming septic shock, the bacterial pathogen is often challenging to identify accurately and in a timely manner. A test that provides higher reliability and shorter turnaround than current practice is urgently needed. Eventual clinical application of U-dHRM will lead to a reduction in unnecessary antibiotic exposure, prevention of untoward side effects and global antibiotic resistance, better antibiotic stewardship, improved and faster diagnostic accuracy, and overall improved pediatric outcomes. In cases of serious bloodstream infection, it could save lives.”
Universal digital high resolution melt analysis for the diagnosis of bacteremia
April Aralar,a Tyler Goshia,a Nanda Ramchandar,b,c Shelley M. Lawrence,d Aparajita Karmakar,e Ankit Sharma,e Mridu Sinha,e David T. Pride,f Peiting Kuo,f Khrissa Lecrone,f Megan Chiu,f Karen Mestan,g Eniko Sajti,g Michelle Vanderpool,h Sarah Lazar,g Melanie Crabtree,g Yordanos Tesfai,g Stephanie I. Fraleya*#
aDepartment of Bioengineering, University of California, San Diego, La Jolla, CA, USA
bDepartment of Pediatrics, Naval Medical Center San Diego, San Diego, CA, USA
cDepartment of Pediatrics, Division of Infectious Diseases, University of California, San Diego, La Jolla, CA, USA
dDepartment of Pediatrics, Division of Neonatology, The University of Utah, Salt Lake City, UT, USA
eMelio, Inc, Santa Clara, CA, USA
fDepartment of Pathology, University of California, San Diego, La Jolla, CA, USA
gDepartment of Pediatrics, Division of Neonatology, University of California, San Diego, La Jolla, CA, USA
hDepartment of Pathology and Laboratory Medicine, Rady Children’s Hospital – San Diego, San Diego, San Diego, CA, USA
Journal: Journal of Molecular Diagnostics
Method of Research: Experimental study
Subject of Research: People
Article Title: Universal digital high resolution melt analysis for the diagnosis of bacteremia
Article Publication Date: 21-Feb-2024
Media Contact
Ioana Patringenaru
University of California – San Diego
ipatring@ucsd.edu
Office: 858-822-0899
All latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

Retinoblastoma: Eye-Catching Investigation into Retinal Tumor Cells
A research team from the Medical Faculty of the University of Duisburg-Essen and the University Hospital Essen has developed a new cell culture model that can be used to better…

A Job Well Done: How Hiroshima’s Groundwater Strategy Helped Manage Floods
Groundwater and multilevel cooperation in recovery efforts mitigated water crisis after flooding. Converting Disasters into Opportunities Society is often vulnerable to disasters, but how humans manage during and after can…

Shaping the Future: DNA Nanorobots That Can Modify Synthetic Cells
Scientists at the University of Stuttgart have succeeded in controlling the structure and function of biological membranes with the help of “DNA origami”. The system they developed may facilitate the…



