New sensor grids record human brain signals in record-breaking resolution
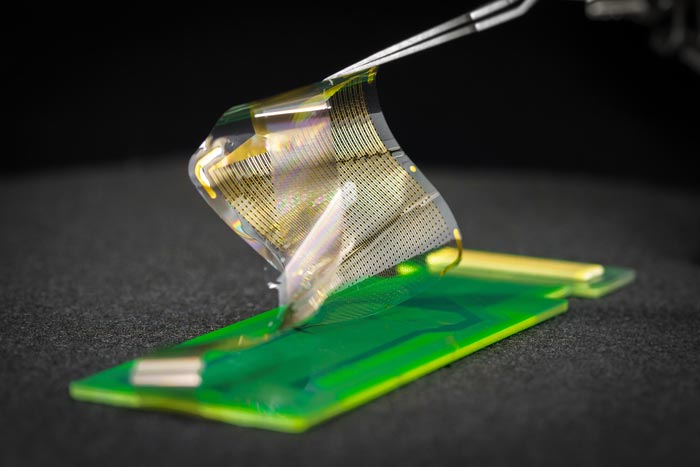
A new array of brain sensors can record electrical signals directly from the surface of the human brain in record-breaking detail. The new brain sensors feature thin, flexible and densely packed grids of either 1,024 or 2,048 embedded electrocorticography (ECoG) sensors.
Credit: David Baillot / UC San Diego Jacobs School of Engineering
High-resolution recordings of electrical signals from the surface of the brain could improve surgeons’ ability to remove brain tumors and treat epilepsy, and could open up new possibilities for medium- and longer- term brain-computer interfaces.
A team of engineers, surgeons and medical researchers has published data from both humans and rats demonstrating that a new array of brain sensors can record electrical signals directly from the surface of the human brain in record-breaking detail. The new brain sensors feature densely packed grids of either 1,024 or 2,048 embedded electrocorticography (ECoG) sensors. The paper was published by the journal Science Translational Medicine on January 19, 2022.
These thin, pliable grids of ECoG sensors, if approved for clinical use, would offer surgeons brain-signal information directly from the surface of the brain’s cortex in 100 times higher resolution than what is available today. Access to this highly detailed perspective on which specific areas of the tissue at the brain’s surface, or cerebral cortex, are active, and when, could provide better guidance for planning surgeries to remove brain tumors and surgically treat drug-resistant epilepsy. Longer term, the team is working on wireless versions of these high resolution ECoG grids that could be used for up to 30 days of brain monitoring for people with intractable epilepsy. The technology also holds potential for permanent implantation to improve the quality of life of people who live with paralysis or other neurodegenerative diseases that can be treated with electrical stimulation such as in Parkinson’s disease, essential tremor, and the neurological movement disorder called dystonia.
The project is led by electrical engineering professor Shadi Dayeh at the University of California San Diego Jacobs School of Engineering. The team of engineers, surgeons and medical researchers hails from UC San Diego; Massachusetts General Hospital; and Oregon Health & Science University.
Next-generation electrocorticography
Recording brain activity from grids of sensors placed directly on the surface of the brain – electrocorticography (ECoG) – is already in common use as a tool by surgeons performing procedures to remove brain tumors and treat epilepsy in people who do not respond to drugs or other treatments. The new work in Science Translational Medicine provides a wide range of peer-reviewed data demonstrating that grids with 1,024 or 2,048 sensors can be used to reliably record and process electrical signals directly from the surface of the brain in both humans and rats. For comparison, the ECoG grids most commonly used in surgeries today typically have between 16 and 64 sensors, although research grade grids with 256 sensors can be custom made.
Being able to record brain signals at such high resolution could improve surgeons’ ability to remove as much of a brain tumor as possible while minimizing damage to healthy brain tissue. In the case of epilepsy, higher resolution brain-signal recording capacity could improve a surgeon’s ability to precisely identify the regions of the brain where the epileptic seizures are originating, so that these regions can be removed without touching nearby brain regions not involved in seizure initiation. In this way, these high resolution grids may enhance preservation of normal, functioning brain tissue.
Demonstrating that ECoG grids with sensors in the thousands function well also opens new opportunities in neuroscience for uncovering a deeper understanding of how the human brain functions. Basic science advances, in turn, could lead to improved treatments grounded in enhanced understanding of brain function.
One millimeter vs one centimeter spacing
Recording brain signals at higher resolution is attributable to the team’s ability to place individual sensors significantly closer to each other without creating problematic interference between nearby sensors. For example, the team’s three-centimeter-by-three-centimeter grid with 1,024-sensors recorded signals directly from the brain tissue of 19 people who agreed to participate in this project during the “downtime” of their already scheduled brain surgeries related to either cancer or epilepsy. In this grid configuration, the sensors are one millimeter apart from each other. In contrast, the ECoG grids already approved for clinical use typically have sensors that are placed one centimeter apart. This provides the new grids 100 sensors per unit area compared to 1 sensor per unit area for clinically used grids, offering 100 times better spatial resolution in interpreting brain signals.
Sensors made with platinum nano-rods
While using platinum-based sensors for recording electrical activity from neurons in the brain is not new, the research team is using platinum in a novel way: nanoscale platinum rods. The nano-rod shape offers more sensing surface area than flat platinum sensors, which helps to make the sensors more sensitive. The sensing system is based on the fact that the electron count in the platinum nano-rods changes in response to neurons firing in the brain.
Charged ions move in and out of a neuron when it fires. This movement of charged ions causes changes in the voltage potential in the cerebrospinal fluid that the neurons are bathed in. These changes in voltage potential in the brain tissue and cerebrospinal fluid change the population count of electrons in the platinum nano-rods by charge screening processes. In this way, the firing of neurons at or near the surface of the cerebral cortex is recorded by the platinum nanorods in near real time and with high precision.
Thin and pliable grids of sensors in various sizes
The human brain is always moving. With each heartbeat, for example, the brain moves with the pulsating blood flowing through it. The platinum nano-rod based sensor grids are thinner and more flexible than today’s clinically approved ECoG grids. The thinness and flexibility allows the sensor grids to move with the brain, enabling a closer connection and better readings. In addition, the grids are manufactured with small, ring-shaped holes that allow cerebral spinal fluid to pass through. In this way, these perfusion holes support a better interface between the sensor grid and the brain surface by allowing the sensor to easily and safely displace the fluid.
The new platinum nano-rod brain sensor grids are ten micrometers thick, approximately one tenth the size of a human hair and 100 times thinner than the one millimeter thick and clinically approved ECoG grids. The nano-rods are embedded in a transparent, soft and flexible biocompatible material called parylene which is in direct contact with the surface of the brain. The electrical signals travel from the brain, through the cerebrospinal fluid, and reach the exposed surfaces of the platinum nano-rods which are recessed within the parylene. This design yields a sensor grid that forms a close and stable connection with the surface of the brain, improving signal quality.
The manufacturing process used also allows for a wide variety of sizes and shapes, which opens up new possibilities for greater and more customized cortical coverage. Collecting signals from larger areas of the brain at the same time could unlock more of the brain’s mysteries.
Through close collaboration between engineers led by electrical engineering professor Dayeh at UC San Diego and clinicians led by neurosurgeon Ahmed Raslan at Oregon Health & Science University, the team has implemented design improvements geared specifically for clinical use. For example, customized sensor grids can be printed with specialized holes that allow surgeons to insert probes at exactly the right spot and apply electrical stimulation directly to brain tissue at specific locations. With the goal of getting higher-resolution ECoG grids approved for clinical use, Dayeh, Raslan and co-first author Youngbin Tchoe have co-founded a startup called Precision Neurotek Inc.
More precise functional mapping
One of the challenges of removing brain tumors is that the presence of the tumor triggers changes in the brain, including changing what areas of the brain are involved in what functions. These changes make it critical for the surgical team to make a personalized map of the patient’s brain – “functional maps” – in order to decide where to cut and where not to cut while removing as much of the tumor as possible.
The authors of the Science Translational Medicine paper demonstrated that these functional maps can be made extremely precise using their platinum nano-rod ECoG sensors. In particular, the team developed functional maps in four different people of a boundary in the brain called the central sulcus. The central sulcus divides the brain’s somatomotor cortex from the somatosensory cortex. In these four individuals, the researchers placed the platinum nano-rod grids on the surfaces of the subjects’ brains and asked them to do a number of activities, including hand grasping. With this information, the researchers reconstructed the actual location of this key landmark in the brain as well as the neural correlates in the brain that correspond to finger sensation and hand grasping. The results from the platinum nano-rod grids aligned with the results from the lower-resolution ECoG grids already approved for clinical use, but with more precision about exactly where this critical functional boundary is between the somatomotor cortex and the somatosensory cortex. The newly delineated curvilinear functional boundary unique to each patient’s brain is superior to the often extrapolated and linear boundary that is determined from today’s one-centimeter-spaced clinical grids.
Neuroscience insights
Some of the team’s newly published data from studies in rats also demonstrate the utility of the grids for opening new avenues in fundamental neuroscience research. The Science Translational Medicine paper, for example, includes what the researchers believe is the first mapping of a cortical column in a rat from brain-surface recordings. In the past, the mapping of cortical columns has only been done by placement of an individual needle at the surface of the brain and sequential electrical stimulation and movement of the needle across the brain surface. More generally, the fact that the platinum nano-rod grids provide high resolution data in both time and space opens up many new possibilities for uncovering new knowledge about how the brain works.
Another observation enabled by the new grids is uncovering the short and local as well as long and broad range brain waves associated with brain function all at the same time. This high- spatial and time-varying (dynamic) picture of the brain activity was documented in several supplementary movies associated with the Science Translational Medicine paper and was used to interpret hand motion in new ways utilizing brain wave patterns.
Next steps
The team is working on a range of initiatives in parallel to advance these grids so that they are eligible for review for approval for short-, medium- and longer-term use. For example, the team, led by UC San Diego electrical engineering professor Shadi Dayeh, was awarded a $12.25 Million NIH grant focused on developing the sensing system to the point that the next step will be a clinical trial for people with treatment-resistant epilepsy. This grant also funds efforts to make the system wireless, which would be important for medium- and longer- term use.
Collaborations
The project is led by electrical engineering professor Shadi Dayeh at the University of California San Diego Jacobs School of Engineering. The team of engineers, surgeons and medical researchers hails from UC San Diego; Massachusetts General Hospital; and Oregon Health & Science University, where the majority of the human surgeries were performed by neurosurgery professor Ahmed Raslan. In addition to the Dayeh-Raslan collaboration, the development of the grids has spanned many years of collaboration between Dayeh and clinical partners spearheaded by Daniel Cleary of UC San Diego, and by Angelique Paulk and Jimmy Yang of Massachusetts General Hospital, and under the supervision of Sharona Ben-Haim and Eric Halgren of UC San Diego and Sydney Cash of Massachusetts General Hospital, and by Brittany Stedelin of Oregon Health & Science University under Raslan’s supervision. Scaling of the grids and their interconnects were led by Dayeh’s postdoctoral fellow Youngbin Tchoe and graduate student Andrew Bourhis who is co-advised by Dayeh and Ian Galton of UC San Diego.
Technology transfer
UC San Diego has filed multiple patent applications on the manufacture of the multi-thousand channel PtNRGrids. Shadi Dayeh, Ahmed Raslan, and Youngbin Tchoe co-founded Precision Neurotech Inc. to support the commercialization of the PtNRGrids.
Funding
This work was supported by the National Institutes of Health Awards No. NBIB DP2-EB029757, NINDS R01NS123655-01, NINDS UG3NS123723-01 and NIDA R01-DA050159 and the National Science Foundation (NSF) Awards No. 1728497 and CAREER No. 1351980 to S.A.D., F32 postdoctoral fellowship to D.C. award # MH120886-01, and an NSF Graduate Research Fellowship Program No. DGE-1650112 to A.M.B.
Authors and Institutions
Co-First authors
Youngbin Tchoe, Andrew M. Bourhis, and Daniel R. Cleary from the Integrated Electronics and Biointerfaces Laboratory, Department of Electrical and Computer Engineering at the University of California San Diego Jacobs School of Engineering. Daniel Cleary is an MD/PhD whose primary appointment is in the Department of Neurological Surgery at UC San Diego Health.
Corresponding author
Shadi A. Dayeh, who leads the Integrated Electronics and Biointerfaces Laboratory, Department of Electrical and Computer Engineering at the University of California San Diego Jacobs School of Engineering
Full Article Author List (by institution)
University of California San Diego
Youngbin Tchoe, Andrew M. Bourhis, Daniel R. Cleary, Jihwan Lee, Karen J. Tonsfeldt, Hongseok Oh, Yun Goo Ro, Keundong Lee, Samantha Russman, Mehran Ganji, Ian Galton, Sharona Ben-Haim, and Shadi A. Dayeh
Oregon Health & Science University
Brittany Stedelin, Erik C. Brown, Dominic Siler and Ahmed M. Raslan
Massachusetts General Hospital
Angelique C. Paulk, and Jimmy C. Yang
Journal: Science Translational Medicine
Method of Research: Experimental study
Subject of Research: People
Article Title: Human brain mapping with multithousand-channel PtNRGrids resolves spatiotemporal dynamics
Article Publication Date: 19-Jan-2022
All latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

Largest magnetic anisotropy of a molecule measured at BESSY II
At the Berlin synchrotron radiation source BESSY II, the largest magnetic anisotropy of a single molecule ever measured experimentally has been determined. The larger this anisotropy is, the better a…

Breaking boundaries: Researchers isolate quantum coherence in classical light systems
LSU quantum researchers uncover hidden quantum behaviors within classical light, which could make quantum technologies robust. Understanding the boundary between classical and quantum physics has long been a central question…

MRI-first strategy for prostate cancer detection proves to be safe
Active monitoring is a sufficiently safe option when prostate MRI findings are negative. There are several strategies for the early detection of prostate cancer. The first step is often a…



