Unlocking richer intracellular recordings
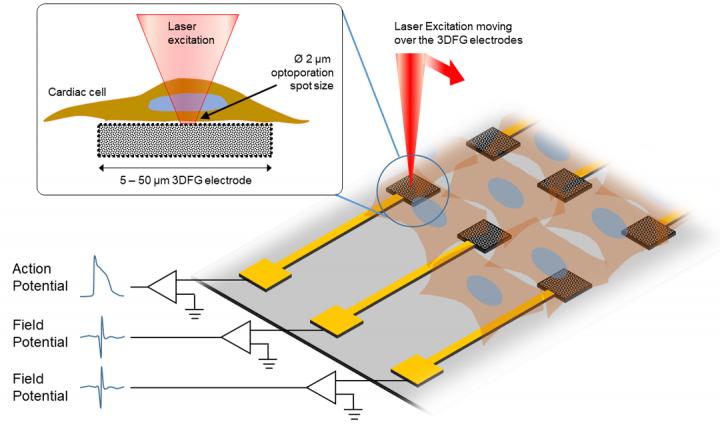
This sketch displays the experimental procedure of ultra-fast laser moving over the 3DFG electrodes.
Credit: College of Engineering, Carnegie Mellon University
CMU/ITT researchers develop novel microelectrode platform to access and record intracellular activity.
Behind every heartbeat and brain signal is a massive orchestra of electrical activity. While current electrophysiology observation techniques have been mostly limited to extracellular recordings, a forward-thinking group of researchers from Carnegie Mellon University and Istituto Italiano di Tecnologia has identified a flexible, low-cost, and biocompatible platform for enabling richer intracellular recordings.
The group’s unique “across the ocean” partnership started two years ago at the Bioelectronics Winter School (BioEl) with libations and a bar napkin sketch. It has evolved into research published today in Science Advances, detailing a novel microelectrode platform that leverages three-dimensional fuzzy graphene (3DFG) to enable richer intracellular recordings of cardiac action potentials with high signal to noise ratio. This advancement could revolutionize ongoing research related to neurodegenerative and cardiac diseases, as well as the development of new therapeutic strategies.
A key leader in this work, Tzahi Cohen-Karni, associate professor of biomedical engineering and materials science and engineering, has studied the properties, effects, and potential applications of graphene throughout his entire career. Now, he is taking a collaborative step in a different direction, using a vertically-grown orientation of the extraordinary carbon-based material (3DFG) to access the intracellular compartment of the cell and record intracellular electrical activity.
Due to its unique electrical properties, graphene stands out as a promising candidate for carbon-based biosensing devices. Recent studies have shown the successful deployment of graphene biosensors for monitoring the electrical activity of cardiomyocytes, or heart cells, outside of the cells, or in other words, extracellular recordings of action potentials. Intracellular recordings, on the other hand, have remained limited due to ineffective tools…until now.
“Our aim is to record the whole orchestra–to see all the ionic currents that cross the cell membrane–not just the subset of the orchestra shown by extracellular recordings,” explains Cohen-Karni. “Adding the dynamic dimension of intracellular recordings is fundamentally important for drug screening and toxicity assay, but this is just one important aspect of our work.”
“The rest is the technology advancement,” Cohen-Karni continues. “3DFG is cheap, flexible and an all-carbon platform; no metals involved. We can generate wafer-sized electrodes of this material to enable multi-site intracellular recordings in a matter of seconds, which is a significant enhancement from an existing tool, like a patch clamp, which requires hours of time and expertise.”
So, how does it work? Leveraging a technique developed by Michele Dipalo and Francesco De Angelis, researchers at Istituto Italiano di Tecnologia, an ultra-fast laser is used to access the cell membrane. By shining short pulses of laser onto the 3DFG electrode, an area of the cell membrane becomes porous in a way, allowing for electrical activity within the cell be recorded. Then, the cardiomyocytes are cultured to further investigate interactions between the cells.
Interestingly, 3DFG is black and absorbs most of the light, resulting in unique optical properties. Combined with its foam-like structure and enormous exposed surface area, 3DFG has many desirable traits that are needed to make small biosensors.
“We have developed a smarter electrode; an electrode that allows us better access,” emphasizes Cohen-Karni. “The biggest advantage from my end is that we can have access to this signal richness, to be able to look into processes of intracellular importance. Having a tool like this will revolutionize the way we can investigate effects of therapeutics on terminal organs, such as the heart.”
As this work moves forward, the team plans to apply its learnings in large-scale cell/tissue interfaces, to better understand tissue development and toxicity of chemical compounds (e.g. drug toxicity).
All latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

Innovative 3D printed scaffolds offer new hope for bone healing
Researchers at the Institute for Bioengineering of Catalonia have developed novel 3D printed PLA-CaP scaffolds that promote blood vessel formation, ensuring better healing and regeneration of bone tissue. Bone is…

The surprising role of gut infection in Alzheimer’s disease
ASU- and Banner Alzheimer’s Institute-led study implicates link between a common virus and the disease, which travels from the gut to the brain and may be a target for antiviral…

Molecular gardening: New enzymes discovered for protein modification pruning
How deubiquitinases USP53 and USP54 cleave long polyubiquitin chains and how the former is linked to liver disease in children. Deubiquitinases (DUBs) are enzymes used by cells to trim protein…



