Individual impurity atoms detectable in graphene
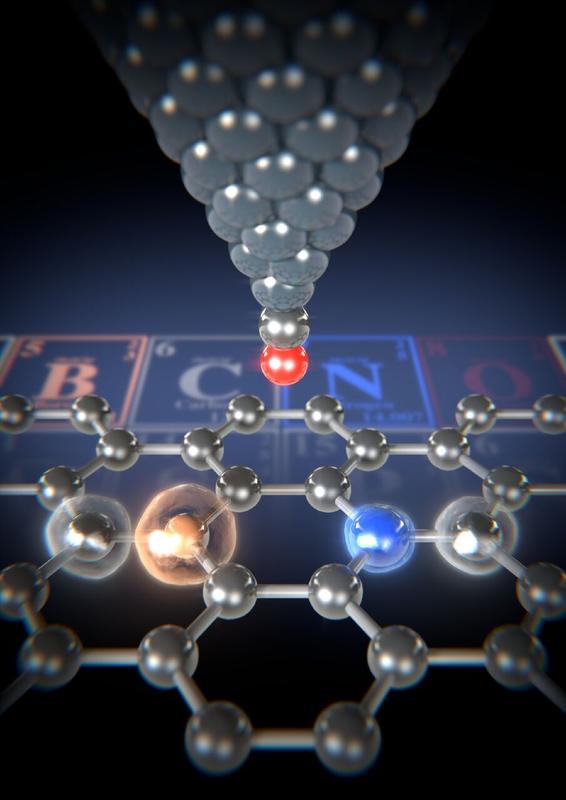
Using the atomic force microscope’s carbon monoxide functionalized tip (red/silver), the forces between the tip and the various atoms in the graphene ribbon can be measured. Image: University of Basel, Department of Physics
Graphene is made of a two-dimensional layer of carbon atoms arranged in a hexagonal lattice. The strong bonds between the carbon atoms make graphene extremely stable yet flexible. It is also an excellent electrical conductor through which electricity can flow with almost no loss.
Graphene’s distinctive properties can be further expanded by incorporating impurity atoms in a process known as “doping”. The impurity atoms cause local changes of the conduction that, for example, allow graphene to be used as a tiny transistor and enable the construction of circuits.
Targeted incorporation
In a collaboration between scientists from the University of Basel and the National Institute for Material Science in Tsukuba in Japan, Kanazawa University and Kwansei Gakuin University in Japan, and Aalto University in Finland, the researchers specifically created and examined graphene ribbons containing impurity atoms.
They replaced particular carbon atoms in the hexagonal lattice with boron and nitrogen atoms using surface chemistry, by placing suitable organic precursor compounds on a gold surface. Under heat exposure up to 400°C, tiny graphene ribbons formed on the gold surface from the precursors, including impurity atoms at specific sites.
Measuring the strength of the atoms
Scientists from the team led by Professor Ernst Meyer from the Swiss Nanoscience Institute and the University of Basel’s Department of Physics examined these graphene ribbons using atomic force microscopy (AFM). They used a carbon monoxide functionalized tip and measured the tiny forces that act between the tip and the individual atoms.
This method allows even the smallest differences in forces to be detected. By looking at the different forces, the researchers were able to map and identify the different atoms. “The forces measured for nitrogen atoms are greater than for a carbon atom,” explains Dr. Shigeki Kawai, lead author of the study and former postdoc in Meyer’s team. “We measured the smallest forces for the boron atoms.” The different forces can be explained by the different proportion of repulsive forces, which is due to the different atomic radii.
Computer simulations confirmed the readings, proving that AFM technology is well-suited to conducting chemical analyses of impurity atoms in the promising two-dimensional carbon compounds.
Original source
Shigeki Kawai, Soichiro Nakatsuka, Takuji Hatakeyama, Rémy Pawlak, Tobias Meier, John Tracey, Ernst Meyer, Adam S. Foster
Multiple heteroatom substitution to graphene nanoribbon
Science Advances (2018), doi: 10.1126/sciadv.aar7181
Further information
Prof. Dr. Ernst Meyer, University of Basel, Department of Physics, tel. +41 61 207 37 24, email: ernst.meyer@unibas.ch
Media Contact
More Information:
http://www.unibas.chAll latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

First-of-its-kind study uses remote sensing to monitor plastic debris in rivers and lakes
Remote sensing creates a cost-effective solution to monitoring plastic pollution. A first-of-its-kind study from researchers at the University of Minnesota Twin Cities shows how remote sensing can help monitor and…

Laser-based artificial neuron mimics nerve cell functions at lightning speed
With a processing speed a billion times faster than nature, chip-based laser neuron could help advance AI tasks such as pattern recognition and sequence prediction. Researchers have developed a laser-based…

Optimising the processing of plastic waste
Just one look in the yellow bin reveals a colourful jumble of different types of plastic. However, the purer and more uniform plastic waste is, the easier it is to…


